Lipid bilayer topology of the transmembrane alpha-helix of M13 Major coat protein and bilayer polarity profile by site-directed fluorescence spectroscopy
- PMID: 15345527
- PMCID: PMC1304553
- DOI: 10.1529/biophysj.104.043208
Lipid bilayer topology of the transmembrane alpha-helix of M13 Major coat protein and bilayer polarity profile by site-directed fluorescence spectroscopy
Abstract
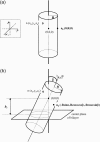
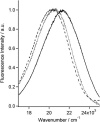
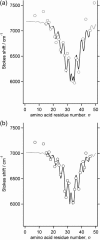
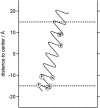
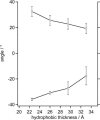
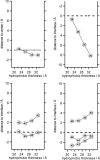
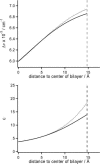
This article presents a new formalism to perform a quantitative fluorescence analysis using the Stokes shift of AEDANS-labeled cysteine mutants of M13 major coat protein incorporated in lipid bilayers. This site-directed fluorescence spectroscopy approach enables us to obtain the topology of the bilayer-embedded transmembrane alpha-helix from the orientation and tilt angles, and relative bilayer location. Both in pure dioleoylphosphatidylcholine and dioleoylphosphatidylcholine/dioleoylphosphatidylglycerol (4:1 mol/mol) bilayers, which have a similar bilayer thickness, the tilt angle of the transmembrane helix of the coat protein turns out to be 23 degrees +/- 4. Upon decreasing the hydrophobic thickness on going from dieicosenoylphosphatidylcholine to dimyristoylphosphatidylcholine, the tilt angle and orientation angle of the transmembrane alpha-helix change. The protein responds to an increase of hydrophobic stress by increasing the tilt angle so as to keep much of its hydrophobic part inside the bilayer. At the same time, the transmembrane helix rotates at its long axis so as to optimize the hydrophobic and electrostatic interactions of the C-terminal phenylalanines and lysines, respectively. The increase of tilt angle cannot completely keep the hydrophobic protein section within the bilayer, but the C-terminal part remains anchored at the acyl-chain/glycerol backbone interface at the cost of the N-terminal section. In addition, our analysis results in the profile of the dielectric constant of the hydrophobic domain of the bilayer. For all phospholipid bilayers studied the profile has a concave shape, with a value of the dielectric constant of 4.0 in the center of the bilayer. The dielectric constant increases on approaching the headgroup region with a value of 12.4 at the acyl-chain/glycerol backbone interface for the various phosphatidylcholines with different chain lengths. For dioleoylphosphatidylcholine/dioleoylphosphatidylglycerol (4:1 mol/mol) bilayers the value of the dielectric constant at the acyl-chain/glycerol backbone interface is 18.6. In conclusion, the consistency of our analysis shows that the applied cysteine-scanning mutagenesis method with AEDANS labeling of a helical transmembrane protein in combination with a quantitative formalism offers a reliable description of the lipid bilayer topology of the protein and bilayer properties. This also indicates that the spacer link between the protein and AEDANS label is long enough to monitor the local polarity of the lipid environment and not that of the amino-acid residues of the protein, and short enough to have the topology of the protein imposing on the fluorescence properties of the AEDANS label.
Figures
Similar articles
-
FRET study of membrane proteins: determination of the tilt and orientation of the N-terminal domain of M13 major coat protein.Biophys J. 2007 Feb 15;92(4):1296-305. doi: 10.1529/biophysj.106.095026. Epub 2006 Nov 17. Biophys J. 2007. PMID: 17114224 Free PMC article.
-
Membrane assembly of M13 major coat protein: evidence for a structural adaptation in the hinge region and a tilted transmembrane domain.Biochemistry. 2004 Nov 9;43(44):13972-80. doi: 10.1021/bi048437x. Biochemistry. 2004. PMID: 15518546
-
A fluorescence method to define transmembrane alpha-helices in membrane proteins: studies with bacterial diacylglycerol kinase.Biochemistry. 2007 Sep 25;46(38):10950-9. doi: 10.1021/bi7008213. Epub 2007 Aug 28. Biochemistry. 2007. PMID: 17722884
-
Anchoring mechanisms of membrane-associated M13 major coat protein.Chem Phys Lipids. 2006 Jun;141(1-2):83-93. doi: 10.1016/j.chemphyslip.2006.02.023. Epub 2006 Mar 29. Chem Phys Lipids. 2006. PMID: 16620800 Review.
-
Bilayer hydrophobic thickness and integral membrane protein function.Curr Protein Pept Sci. 2011 Dec;12(8):760-6. doi: 10.2174/138920311798841681. Curr Protein Pept Sci. 2011. PMID: 22044142 Review.
Cited by
-
Site-directed fluorescence labeling of a membrane protein with BADAN: probing protein topology and local environment.Biophys J. 2008 May 15;94(10):3945-55. doi: 10.1529/biophysj.107.125807. Epub 2008 Jan 30. Biophys J. 2008. PMID: 18234831 Free PMC article.
-
Hydrophobic Mismatch Drives the Interaction of E5 with the Transmembrane Segment of PDGF Receptor.Biophys J. 2015 Aug 18;109(4):737-49. doi: 10.1016/j.bpj.2015.07.022. Biophys J. 2015. PMID: 26287626 Free PMC article.
-
FRET study of membrane proteins: determination of the tilt and orientation of the N-terminal domain of M13 major coat protein.Biophys J. 2007 Feb 15;92(4):1296-305. doi: 10.1529/biophysj.106.095026. Epub 2006 Nov 17. Biophys J. 2007. PMID: 17114224 Free PMC article.
-
Structure of membrane-embedded M13 major coat protein is insensitive to hydrophobic stress.Biophys J. 2007 Nov 15;93(10):3541-7. doi: 10.1529/biophysj.107.112698. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704180 Free PMC article.
-
Lipid chain-length dependence for incorporation of alamethicin in membranes: electron paramagnetic resonance studies on TOAC-spin labeled analogs.Biophys J. 2007 Jun 1;92(11):4002-11. doi: 10.1529/biophysj.107.104026. Epub 2007 Mar 9. Biophys J. 2007. PMID: 17351010 Free PMC article.
References
-
- Binder, H. 2003. The molecular architecture of lipid membranes—new insights from hydration-tuning infrared linear dichroism spectroscopy. Applied Spectrosc. Rev. 38:15–69.
-
- Dumas, F., M. C. Lebrun, and J. F. Tocanne. 1999. Is the protein/lipid hydrophobic matching principle relevant to membrane organization and functions? FEBS Lett. 458:271–277. - PubMed
-
- Elston, T., H. Wang, and G. Oster. 1998. Energy transduction in ATP synthase. Lett. Nature. 391:510–513. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

