Rational design of solution additives for the prevention of protein aggregation
- PMID: 15345542
- PMCID: PMC1304568
- DOI: 10.1529/biophysj.104.042473
Rational design of solution additives for the prevention of protein aggregation
Abstract
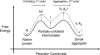
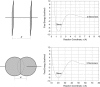
We have developed a statistical-mechanical model of the effect of solution additives on protein association reactions. This model incorporates solvent radial distribution functions obtained from all-atom molecular dynamics simulations of particular proteins into simple models of protein interactions. In this way, the effects of additives can be computed along the entire association/dissociation reaction coordinate. We used the model to test our hypothesis that a class of large solution additives, which we term "neutral crowders," can slow protein association and dissociation by being preferentially excluded from protein-protein encounter complexes, in a manner analogous to osmotic stress. The magnitude of this proposed "gap effect" was probed for two simple model systems: the association of two spheres and the association of two planes. Our results suggest that for a protein of 20 A radius, an 8 A additive can increase the free energy barrier for association and dissociation by as much as 3-6 kcal/mol. Because the proposed gap effect is present only for reactions involving multiple molecules, it can be exploited to develop novel additives that affect protein association reactions although having little or no effect on unimolecular reactions such as protein folding. This idea has many potential applications in areas such as the stabilization of proteins against aggregation during folding and in pharmaceutical formulations.
Figures


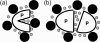

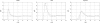


Similar articles
-
Role of arginine in the stabilization of proteins against aggregation.Biochemistry. 2005 Mar 29;44(12):4919-25. doi: 10.1021/bi047528r. Biochemistry. 2005. PMID: 15779919
-
Effects of macromolecular crowding on biochemical reaction equilibria: a molecular thermodynamic perspective.Biophys J. 2007 Sep 1;93(5):1464-73. doi: 10.1529/biophysj.107.104646. Epub 2007 May 18. Biophys J. 2007. PMID: 17513384 Free PMC article.
-
Quantifying kinetic paths of protein folding.Biophys J. 2005 Sep;89(3):1612-20. doi: 10.1529/biophysj.104.055186. Epub 2005 Jul 1. Biophys J. 2005. PMID: 15994895 Free PMC article.
-
Conformational stability and folding mechanisms of dimeric proteins.Prog Biophys Mol Biol. 2008 Sep;98(1):61-84. doi: 10.1016/j.pbiomolbio.2008.05.004. Epub 2008 Jun 8. Prog Biophys Mol Biol. 2008. PMID: 18602415 Review.
-
Thermal stability of proteins.Ann N Y Acad Sci. 2005 Dec;1066:12-33. doi: 10.1196/annals.1363.003. Ann N Y Acad Sci. 2005. PMID: 16533916 Review.
Cited by
-
Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods.Molecules. 2020 Oct 21;25(20):4854. doi: 10.3390/molecules25204854. Molecules. 2020. PMID: 33096797 Free PMC article. Review.
-
Ternary system of solution additives with arginine and salt for refolding of beta-galactosidase.Protein J. 2010 Apr;29(3):161-6. doi: 10.1007/s10930-010-9235-7. Protein J. 2010. PMID: 20213119
-
Self-aggregation of a recombinant form of the propeptide NH2-terminal of the precursor of pulmonary surfactant protein SP-B: a conformational study.J Ind Microbiol Biotechnol. 2008 Nov;35(11):1367-76. doi: 10.1007/s10295-008-0437-3. Epub 2008 Sep 17. J Ind Microbiol Biotechnol. 2008. PMID: 18797948
-
Inhibition of protein aggregation: supramolecular assemblies of arginine hold the key.PLoS One. 2007 Nov 14;2(11):e1176. doi: 10.1371/journal.pone.0001176. PLoS One. 2007. PMID: 18000547 Free PMC article.
-
Ion-specific effects on prion nucleation and strain formation.J Biol Chem. 2013 Oct 18;288(42):30300-30308. doi: 10.1074/jbc.M113.467829. Epub 2013 Aug 29. J Biol Chem. 2013. PMID: 23990463 Free PMC article.
References
-
- Arakawa, T., and S. N. Timasheff. 1982. Stabilization of protein structure by sugars. Biochemistry. 21:6536–6544. - PubMed
-
- Arakawa, T., and K. Tsumoto. 2003. The effects of arginine on refolding of aggregated proteins: not facilitate refolding, but suppress aggregation. Biochem. Biophys. Res. Commun. 304:148–152. - PubMed
-
- Arora, D., and N. Khanna. 1996. Method for increasing the yield of properly folded recombinant human γ-interferon from inclusion bodies. J. Biotechnol. 52:127–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

