Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway
- PMID: 15345546
- PMCID: PMC1304572
- DOI: 10.1529/biophysj.104.043174
Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway
Abstract
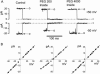
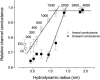
The newly proposed function of the maxi-anion channel as a conductive pathway for ATP release requires that its pore is sufficiently large to permit passage of a bulky ATP(4-) anion. We found a linear relationship between relative permeability of organic anions of different size and their relative ionic mobility (measured as the ratio of ionic conductance) with a slope close to 1, suggesting that organic anions tested with radii up to 0.49 nm (lactobionate) move inside the channel by free diffusion. In the second approach, we, for the first time, succeeded in pore sizing by the nonelectrolyte exclusion method in single-channel patch-clamp experiments. The cutoff radii of PEG molecules that could access the channel from intracellular (1.16 nm) and extracellular (1.42 nm) sides indicated an asymmetry of the two entrances to the channel pore. Measurements by symmetrical two-sided application of PEG molecules yielded an average functional pore radius of approximately 1.3 nm. These three estimates are considerably larger than the radius of ATP(4-) (0.57-0.65 nm) and MgATP(2-) (approximately 0.60 nm). We therefore conclude that the nanoscopic maxi-anion channel pore provides sufficient room to accommodate ATP and is well suited to its function as a conductive pathway for ATP release in cell-to-cell communication.
Figures

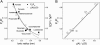



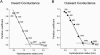
References
-
- Bahamonde, M. I., J. M. Fernandez-Fernandez, F. X. Guix, E. Vazquez, and M. A. Valverde. 2003. Plasma membrane voltage-dependent anion channel mediates anti-estrogen-activated maxi Cl− currents in C1300 neuroblastoma cells. J. Biol. Chem. 278:33284–33289. - PubMed
-
- Bahamonde, M. I., and M. A. Valverde. 2003. Voltage-dependent anion channel localises to the plasma membrane and peripheral but not perinuclear mitochondria. Pflugers Arch. 446:309–313. - PubMed
-
- Bathori, G., I. Parolini, F. Tombola, I. Szabo, A. Messina, M. Oliva, V. De Pinto, M. Lisanti, M. Sargiacomo, and M. Zoratti. 1999. Porin is present in the plasma membrane where it is concentrated in caveolae and caveolae-related domains. J. Biol. Chem. 274:29607–29612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

