The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior
- PMID: 15345551
- PMCID: PMC1304577
- DOI: 10.1529/biophysj.104.044529
The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior
Abstract
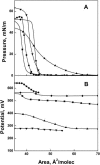
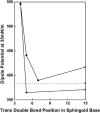
The biological activities of ceramides show a large variation with small changes in molecular structure. To help understand how the structure regulates the activity of this important lipid second messenger, we investigated the interfacial features of a series of synthetic ceramide analogs in monomolecular films at the argon-buffer interface. To minimize differences arising from the N-acyl moiety, each analog had either a N-hexadecanoyl or a N-cis-4-hexadecenoyl moiety amide linked to the nitrogen of the sphingosine backbone. We found that the trans 4,5-unsaturation in the sphingosine backbone promoted closer packing and lower compressibilities of ceramide analogs in interfaces relative to comparable saturated species. Moreover, structures with this feature exhibited dipole potentials as much as 150-250 mV higher than comparable compounds lacking 4,5-unsaturation. The results support the hypothesis by M.C. Yappert and co-workers that trans unsaturation in the vicinity of C4 of the sphingoid backbone augments intramolecular hydration/hydrogen bonding in the polar region. This intramolecular hydration may allow the close packing of the ceramide molecules and engender their high dipole potentials. These properties of ceramides and their analogs may be important determinants of biological function.
Figures



References
-
- Abrahamsson, S., B. Dahlén, H. Löfgren, and I. Pascher. 1978. Lateral packing of hydrocarbon chains. Prog. Chem. Fats Other Lipids. 16:125–143. - PubMed
-
- Bielawska, A., H. M. Crane, D. Liotta, L. M. Obeid, and Y. A. Hannun. 1993. Selectivity of ceramide-mediated biology. Lack of activity of erythro-dihydroceramide. J. Biol. Chem. 268:26226–26232. - PubMed
-
- Brockman, H. L. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73:57–79. - PubMed
-
- Brockman, H. L. 1999. Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr. Opin. Struct. Biol. 9:438–443. - PubMed
-
- Brockman, H. L., J. M. Smaby, and D. E. Jarvis. 1984. Automation of surface cleaning and sample addition for surface balances. J. Phys. E: Sci. Instrum. 17:351–353.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

