Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study
- PMID: 15345554
- PMCID: PMC1304580
- DOI: 10.1529/biophysj.104.044552
Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study
Abstract
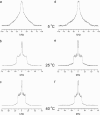
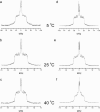
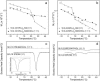
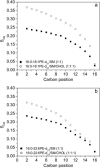
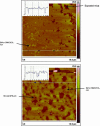
We have previously suggested that the omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA) may in part function by enhancing membrane lipid phase separation into lipid rafts. Here we further tested for differences in the molecular interactions of an oleic (OA) versus DHA-containing phospholipid with sphingomyelin (SM) and cholesterol (CHOL) utilizing (2)H NMR spectroscopy, differential scanning calorimetry, atomic force microscopy, and detergent extractions in model bilayer membranes. (2)H NMR and DSC (differential scanning calorimetry) established the phase behavior of the OA-containing 1-[(2)H(31)]palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (16:0-18:1PE-d(31))/SM (1:1) and the DHA-containing 1-[(2)H(31)]palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphoethanolamine (16:0-22:6PE-d(31))/SM (1:1) in the absence and presence of equimolar CHOL. CHOL was observed to affect the OA-containing phosphatidylethanolamine (PE) more than the DHA-containing PE, as exemplified by >2 x greater increase in order measured for the perdeuterated palmitic chain in 16:0-18:1PE-d(31)/SM (1:1) compared to 16:0-22:6PE-d(31)/SM (1:1) bilayers in the liquid crystalline phase. Atomic force microscopy (AFM) experiments showed less lateral phase separation between 16:0-18:1PE-rich and SM/CHOL-rich raft domains in 16:0-18:1PE/SM/CHOL (1:1:1) bilayers than was observed when 16:0-22:6PE replaced 16:0-18:1PE. Differences in the molecular interaction of 16:0-18:1PE and 16:0-22:6PE with SM/CHOL were also found using biochemical detergent extractions. In the presence of equimolar SM/CHOL, 16:0-18:1PE showed decreased solubilization in comparison to 16:0-22:6PE, indicating greater phase separation with the DHA-PE. Detergent experiments were also conducted with cardiomyocytes fed radiolabeled OA or DHA. Although both OA and DHA were found to be largely detergent solubilized, the amount of OA that was found to be associated with raft-rich detergent-resistant membranes exceeded DHA by almost a factor of 2. We conclude that the OA-PE phase separates from rafts far less than DHA-PE, which may have implications for cellular signaling.
Figures




References
-
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry. 36:10944–10953. - PubMed
-
- Aussenac, F., M. Tavares, and E. J. Dufourc. 2003. Cholesterol dynamics in membranes of raft composition: a molecular point of view from 2H and 31P solid-state NMR. Biochemistry. 42:1383–1390. - PubMed
-
- Brown, D. A., and E. London. 2000. Structure and function of sphingolipid and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. - PubMed
-
- Brzustowicz, M. R., V. Cherezov, M. Zerouga, M. Caffrey, W. Stillwell, and S. R. Wassall. 2002a. Controlling membrane cholesterol content. A role for polyunsaturated (docosahexaenoate) phospholipids. Biochemistry. 41:12509–12519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

