Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling
- PMID: 15345557
- PMCID: PMC1304583
- DOI: 10.1529/biophysj.103.039131
Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling
Abstract
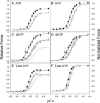
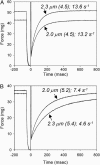
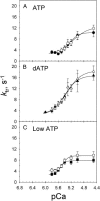
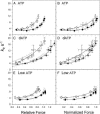
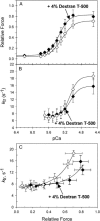
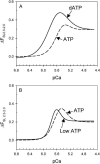
We examined the influence of cross-bridge cycling kinetics on the length dependence of steady-state force and the rate of force redevelopment (k(tr)) during Ca(2+)-activation at sarcomere lengths (SL) of 2.0 and 2.3 microm in skinned rat cardiac trabeculae. Cross-bridge kinetics were altered by either replacing ATP with 2-deoxy-ATP (dATP) or by reducing [ATP]. At each SL dATP increased maximal force (F(max)) and Ca(2+)-sensitivity of force (pCa(50)) and reduced the cooperativity (n(H)) of force-pCa relations, whereas reducing [ATP] to 0.5 mM (low ATP) increased pCa(50) and n(H) without changing F(max). The difference in pCa(50) between SL 2.0 and 2.3 microm (Delta pCa(50)) was comparable between ATP and dATP, but reduced with low ATP. Maximal k(tr) was elevated by dATP and reduced by low ATP. Ca(2+)-sensitivity of k(tr) increased with both dATP and low ATP and was unaffected by altered SL under all conditions. Significantly, at equivalent levels of submaximal force k(tr) was faster at short SL or increased lattice spacing. These data demonstrate that the SL dependence of force depends on cross-bridge kinetics and that the increase of force upon SL extension occurs without increasing the rate of transitions between nonforce and force-generating cross-bridge states, suggesting SL or lattice spacing may modulate preforce cross-bridge transitions.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

