Incoherent manipulation of the photoactive yellow protein photocycle with dispersed pump-dump-probe spectroscopy
- PMID: 15345564
- PMCID: PMC1304590
- DOI: 10.1529/biophysj.104.043794
Incoherent manipulation of the photoactive yellow protein photocycle with dispersed pump-dump-probe spectroscopy
Abstract
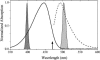
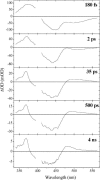
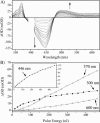
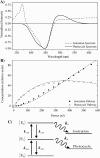
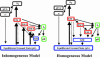
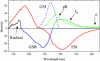
Photoactive yellow protein is the protein responsible for initiating the "blue-light vision" of Halorhodospira halophila. The dynamical processes responsible for triggering the photoactive yellow protein photocycle have been disentangled with the use of a novel application of dispersed ultrafast pump-dump-probe spectroscopy, where the photocycle can be started and interrupted with appropriately tuned and timed laser pulses. This "incoherent" manipulation of the photocycle allows for the detailed spectroscopic investigation of the underlying photocycle dynamics and the construction of a fully self-consistent dynamical model. This model requires three kinetically distinct excited-state intermediates, two (ground-state) photocycle intermediates, I(0) and pR, and a ground-state intermediate through which the protein, after unsuccessful attempts at initiating the photocycle, returns to the equilibrium ground state. Also observed is a previously unknown two-photon ionization channel that generates a radical and an ejected electron into the protein environment. This second excitation pathway evolves simultaneously with the pathway containing the one-photon photocycle intermediates.
Figures



References
-
- Baca, M., G. E. Borgstahl, M. Boissinot, P. M. Burke, D. R. Williams, K. A. Slater, and E. D. Getzoff. 1994. Complete chemical structure of photoactive yellow protein: novel thioester-linked 4-hydroxycinnamyl chromophore and photocycle chemistry. Biochemistry. 33:14369–14377. - PubMed
-
- Baltuška, A., I. H. M. van Stokkum, A. Kroon, R. Monshouwer, K. J. Hellingwerf, and R. van Grondelle. 1997. The primary events in the photoactivation of yellow protein. Chem. Phys. Lett. 270:263–266.
-
- Brudler, R., R. Rammelsberg, T. T. Woo, E. D. Getzoff, and K. Gerwert. 2001. Structure of the I1 early intermediate of photoactive yellow protein by FTIR spectroscopy. Nat. Struct. Biol. 8:265–270. - PubMed
-
- Changenet, P., H. Zhang, M. J. van der Meer, K. J. Hellingwerf, and M. Glasbeek. 1998. Subpicosecond fluorescence upconversion measurements of primary events in yellow proteins. Chem. Phys. Lett. 282:276–282.
-
- Changenet-Barret, P., C. Choma, E. Gooding, W. DeGrado, and R. M. Hochstrasser. 2000. Ultrafast dielectric response of proteins from dynamics stokes shifting of coumarin in calmodulin. J. Phys. Chem. B. 104:9322–9329.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

