Oligomeric beta-structure of the membrane-bound HIV-1 fusion peptide formed from soluble monomers
- PMID: 15345571
- PMCID: PMC1304598
- DOI: 10.1529/biophysj.103.028530
Oligomeric beta-structure of the membrane-bound HIV-1 fusion peptide formed from soluble monomers
Abstract
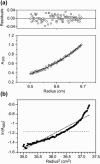
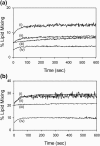
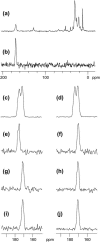
The human immunodeficiency virus type 1 (HIV-1) fusion peptide serves as a useful model system for understanding viral/target cell fusion, at least to the lipid mixing stage. Previous solid-state NMR studies have shown that the peptide adopts an oligomeric beta-strand structure when associated with a lipid and cholesterol mixture close to that of membranes of host cells of the virus. In this study, this structure was further investigated using four different peptide constructs. In aqueous buffer solution, two of the constructs were primarily monomeric whereas the other two constructs had significant populations of oligomers/aggregates. NMR measurements for all membrane-associated peptide constructs were consistent with oligomeric beta-strand structure. Thus, constructs that are monomeric in solution can be converted to oligomers as a result of membrane association. In addition, samples prepared by very different methods had very similar NMR spectra, which indicates that the beta-strand structure is an equilibrium rather than a kinetically trapped structure. Lipid mixing assays were performed to assess the fusogenicities of the different constructs, and there was not a linear correlation between the solution oligomeric state and fusogenicity. However, the functional assays do suggest that small oligomers may be more fusogenic than either monomers or large aggregates.
Figures
References
-
- Afonin, S., R. W. Glaser, M. Berditchevskaia, P. Wadhwani, K. H. Guhrs, U. Mollmann, A. Perner, and A. S. Ulrich. 2003. 4-Fluorophenylglycine as a label for 19F NMR structure analysis of membrane-associated peptides. ChemBioChem. 4:1151–1163. - PubMed
-
- Balbach, J. J., J. Yang, D. P. Weliky, P. J. Steinbach, V. Tugarinov, J. Anglister, and R. Tycko. 2000. Probing hydrogen bonds in the antibody-bound HIV-1 gp120 V3 loop by solid state NMR REDOR measurements. J. Biomol. NMR. 16:313–327. - PubMed
-
- Bennett, A. E., C. M. Rienstra, M. Auger, K. V. Lakshmi, and R. G. Griffin. 1995. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 103:6951–6958.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

