Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization
- PMID: 15345578
- PMCID: PMC1304605
- DOI: 10.1529/biophysj.103.035097
Coordinated behavior of mitochondria in both space and time: a reactive oxygen species-activated wave of mitochondrial depolarization
Abstract
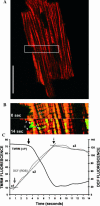
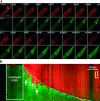
Reactive oxygen species (ROS) can trigger a transient burst of mitochondrial ROS production via ROS activation of the mitochondrial permeability transition pore (MPTP), a phenomenon termed ROS-induced ROS release (RIRR). The goal of this study was to investigate if the generation of ROS in a discrete region of a cardiomyocyte could serve to propagate RIRR-mediated mitochondrial depolarizations throughout a cell. Our experiments revealed that localized RIRR activated either RIRR-mediated fluctuations in mitochondrial membrane potential (time period: 3-10 min) or a traveling wave of depolarization of the cell's mitochondria (velocity: approximately 5 microm/min). Both phenomena appeared to be mediated by the mitochondrial permeability transition pore and eventually encompassed the majority of the mitochondrial population of both isolated rat and rabbit cardiomyocytes. Furthermore, depolarization was often reversible; the waves of depolarization were then followed by a rapid (approximately 40 microm/min) repolarization wave of the mitochondria. We show that the RIRR can function to communicate the mitochondrial permeability transition from one mitochondrion to another in the isolated adult cardiomyocyte.
Figures






References
-
- Aon, M. A., S. Cortassa, E. Maraban, and B. O'Rourke. 2003. Synchronized whole-cell oscixllations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278:44735–44744. - PubMed
-
- Armstrong, J. S., and D. P. Jones. 2002. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 16:1263–1265. - PubMed
-
- Aronis, A., R. Komarnitsky, S. Shilo, and O. Tirosh. 2002. Membrane depolarization of isolated rat liver mitochondria attenuates permeability transition pore opening and oxidant production. Antioxid. Redox. Signal. 4:647–654. - PubMed
-
- Beavis, A. D., and H. Davatol-Hag. 1996. The mitochondrial inner membrane anion channel is inhibited by DIDS. J. Bioenerg. Biomembr. 28:207–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

