Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy
- PMID: 15345595
- PMCID: PMC2553211
- DOI: 10.1182/blood-2004-06-2482
Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy
Abstract
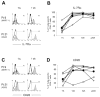
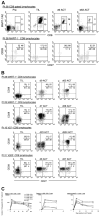
In humans, the pathways of memory T-cell differentiation remain poorly defined. Recently, adoptive cell transfer (ACT) of tumor-reactive T lymphocytes to metastatic melanoma patients after nonmyeloablative chemotherapy has resulted in persistence of functional, tumor-reactive lymphocytes, regression of disease, and induction of melanocyte-directed autoimmunity in some responding patients. In the current study, longitudinal phenotypic analysis was performed on melanoma antigen-specific CD8+ T cells during their transition from in vitro cultured effector cells to long-term persistent memory cells following ACT to 6 responding patients. Tumor-reactive T cells used for therapy were generally late-stage effector cells with a CD27Lo CD28Lo CD45RA- CD62 ligand- (CD62L-) CC chemokine receptor 7- (CCR7-) interleukin-7 receptor alphaLo (IL-7RalphaLo) phenotype. After transfer, rapid up-regulation and continued expression of IL-7Ralpha in vivo suggested an important role for IL-7R in immediate and long-term T-cell survival. Although the tumor antigen-specific T-cell population contracted between 1 and 4 weeks after transfer, stable numbers of CD27+)CD28+ tumor-reactive T cells were maintained, demonstrating their contribution to the development of long-term, melanoma-reactive memory CD8+ T cells in vivo. At 2 months after transfer, melanoma-reactive T cells persisted at high levels and displayed an effector memory phenotype, including a CD27+ CD28+ CD62L- CCR7- profile, which may explain in part their ability to mediate tumor destruction.
Figures




References
-
- Sprent J, Tough DF. T cell death and memory. Science. 2001;293:245–248. - PubMed
-
- Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–223. - PubMed
-
- Kaech SM, Hemby S, Kersh E, et al. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. - PubMed
-
- Kaech SM, Tan JT, Wherry EJ, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

