Heart valve development: endothelial cell signaling and differentiation
- PMID: 15345668
- PMCID: PMC2810618
- DOI: 10.1161/01.RES.0000141146.95728.da
Heart valve development: endothelial cell signaling and differentiation
Abstract
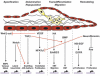
During the past decade, single gene disruption in mice and large-scale mutagenesis screens in zebrafish have elucidated many fundamental genetic pathways that govern early heart patterning and differentiation. Specifically, a number of genes have been revealed serendipitously to play important and selective roles in cardiac valve development. These initially surprising results have now converged on a finite number of signaling pathways that regulate endothelial proliferation and differentiation in developing and postnatal heart valves. This review highlights the roles of the most well-established ligands and signaling pathways, including VEGF, NFATc1, Notch, Wnt/beta-catenin, BMP/TGF-beta, ErbB, and NF1/Ras. Based on the interactions among and relative timing of these pathways, a signaling network model for heart valve development is proposed.
Figures







References
-
- Ferencz C, Rubin J, Loffredo C, Magee C. Epidemiology of congenital heart disease: the Baltimore-Washington Infant Study, 1981–1989. Futura Publishing; Mt. Kisko, New York: 1993.
-
- Loffredo CA. Epidemiology of cardiovascular malformations: prevalence and risk factors. Am J Med Genet. 2000;97:319–325. - PubMed
-
- Bruneau B, Nemer G, Schmitt J, Charron F, Robitaille L, Caron S, Conner D, Gessler M, Nemer M, Seidman C, Seidman J. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. - PubMed
-
- Tartaglia M, Mehler E, Goldberg R, Zampino G, Brunner H, Kremer H, van der Bergt I, Crosby A, Ion A, Jeffery S, Kalidas K, Patton M, Kucherlapati R, Gelb B. Mutations in PTPN11, encoding the protein phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. - PubMed
-
- Pierpont M, Markwald R, Lin A. Genetic aspects of atrioventricular septal defects. Am J Med Genet. 2000;97:289–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

