Reducing chloride conductance prevents hyperkalaemia-induced loss of twitch force in rat slow-twitch muscle
- PMID: 15345748
- PMCID: PMC1665340
- DOI: 10.1113/jphysiol.2004.071498
Reducing chloride conductance prevents hyperkalaemia-induced loss of twitch force in rat slow-twitch muscle
Abstract
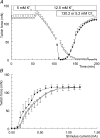
Exercise-induced loss of skeletal muscle K(+) can seriously impede muscle performance through membrane depolarization. Thus far, it has been assumed that the negative equilibrium potential and large membrane conductance of Cl(-) attenuate the loss of force during hyperkalaemia. We questioned this idea because there is some evidence that Cl(-) itself can exert a depolarizing influence on membrane potential (V(m)). With this study we tried to identify the possible roles played by Cl(-) during hyperkalaemia. Isolated rat soleus muscles were kept at 25 degrees C and twitch contractions were evoked by current pulses. Reducing [Cl(-)](o) to 5 mM, prior to introducing 12.5 mM K(o), prevented the otherwise occurring loss of force. Reversing the order of introducing these two solutions revealed an additional effect, i.e. the ongoing hyperkalaemia-related loss of force was sped up tenfold after reducing [Cl(-)](o). However, hereafter twitch force recovered completely. The recovery of force was absent at [K(+)](o) exceeding 14 mM. In addition, reducing [Cl(-)](o) increased membrane excitability by 24%, as shown by a shift in the relationship between force and current level. Measurements of V(m) indicated that the antagonistic effect of reducing [Cl(-)](o) on hyperkalaemia-induced loss of force was due to low-Cl(-)-induced membrane hyperpolarization. The involvement of specific Cl(-) conductance was established with 9-anthracene carboxylic acid (9-AC). At 100 microm, 9-AC reduced the loss of force due to hyperkalaemia, while at 200 microm, 9-AC completely prevented loss of force. To study the role of the Na(+)-K(+)-2Cl(-) cotransporter (NKCC1) in this matter, we added 400 microm of the NKCC inhibitor bumetanide to the incubation medium. This did not affect the hyperkalaemia-induced loss of force. We conclude that Cl(-) exerts a permanent depolarizing influence on V(m). This influence of Cl(-) on V(m), in combination with a large membrane conductance, can apparently have two different effects on hyperkalaemia-induced loss of force. It might exert a stabilizing influence on force production during short periods of hyperkalaemia, but it can add to the loss of force during prolonged periods of hyperkalaemia.
Figures





Similar articles
-
Increased excitability of acidified skeletal muscle: role of chloride conductance.J Gen Physiol. 2005 Feb;125(2):237-46. doi: 10.1085/jgp.200409173. J Gen Physiol. 2005. PMID: 15684096 Free PMC article.
-
Effects of chloride transport on bistable behaviour of the membrane potential in mouse skeletal muscle.J Physiol. 2002 Jul 1;542(Pt 1):181-91. doi: 10.1113/jphysiol.2001.013298. J Physiol. 2002. PMID: 12096060 Free PMC article.
-
Effect of transverse-tubular chloride conductance on excitability in skinned skeletal muscle fibres of rat and toad.J Physiol. 1998 Jun 1;509 ( Pt 2)(Pt 2):551-64. doi: 10.1111/j.1469-7793.1998.551bn.x. J Physiol. 1998. PMID: 9575303 Free PMC article.
-
In isolated skeletal muscle, excitation may increase extracellular K+ 10-fold; how can contractility be maintained?Exp Physiol. 2011 Mar;96(3):356-68. doi: 10.1113/expphysiol.2010.054999. Epub 2010 Dec 1. Exp Physiol. 2011. PMID: 21123362
-
The bumetanide-sensitive Na-K-2Cl cotransporter NKCC1 as a potential target of a novel mechanism-based treatment strategy for neonatal seizures.Neurosurg Focus. 2008 Sep;25(3):E22. doi: 10.3171/FOC/2008/25/9/E22. Neurosurg Focus. 2008. PMID: 18759624 Review.
Cited by
-
Regulation of ClC-1 and KATP channels in action potential-firing fast-twitch muscle fibers.J Gen Physiol. 2009 Oct;134(4):309-22. doi: 10.1085/jgp.200910290. J Gen Physiol. 2009. PMID: 19786584 Free PMC article.
-
Relationship between membrane Cl- conductance and contractile endurance in isolated rat muscles.J Physiol. 2013 Jan 15;591(2):531-45. doi: 10.1113/jphysiol.2012.243246. Epub 2012 Oct 8. J Physiol. 2013. PMID: 23045345 Free PMC article.
-
Increased excitability of acidified skeletal muscle: role of chloride conductance.J Gen Physiol. 2005 Feb;125(2):237-46. doi: 10.1085/jgp.200409173. J Gen Physiol. 2005. PMID: 15684096 Free PMC article.
-
The effect of intracellular acidification on the relationship between cell volume and membrane potential in amphibian skeletal muscle.J Physiol. 2005 Mar 15;563(Pt 3):745-64. doi: 10.1113/jphysiol.2004.079657. Epub 2004 Dec 23. J Physiol. 2005. PMID: 15618273 Free PMC article.
-
Role of physiological ClC-1 Cl- ion channel regulation for the excitability and function of working skeletal muscle.J Gen Physiol. 2016 Apr;147(4):291-308. doi: 10.1085/jgp.201611582. J Gen Physiol. 2016. PMID: 27022190 Free PMC article. Review.
References
-
- Blum R, Westphal W. On the actions of a chloride conductance blocking agent (9-AC) on the resting membrane potential of single mammalian skeletal muscle fibres. Pflugers Arch. 1981;389:45–45.
-
- Bretag AH. Muscle chloride channels. Phys Rev. 1987;67:618–724. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

