Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1
- PMID: 15347586
- PMCID: PMC1304789
- DOI: 10.1529/biophysj.104.048447
Distinct effects on Ca2+ handling caused by malignant hyperthermia and central core disease mutations in RyR1
Abstract
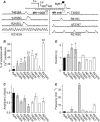
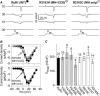
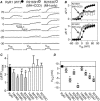
Malignant hyperthermia (MH) and central core disease (CCD) are disorders of skeletal muscle Ca2+ homeostasis that are linked to mutations in the type 1 ryanodine receptor (RyR1). Certain RyR1 mutations result in an MH-selective phenotype (MH-only), whereas others result in a mixed phenotype (MH + CCD). We characterized effects on Ca2+ handling and excitation-contraction (EC) coupling of MH-only and MH + CCD mutations in RyR1 after expression in skeletal myotubes derived from RyR1-null (dyspedic) mice. Compared to wild-type RyR1-expressing myotubes, MH + CCD- and MH-only-expressing myotubes exhibited voltage-gated Ca2+ release (VGCR) that activated at more negative potentials and displayed a significantly higher incidence of spontaneous Ca2+ oscillations. However, maximal VGCR was reduced only for MH + CCD mutants (Y4795C, R2435L, and R2163H) in which spontaneous Ca2+ oscillations occurred with significantly longer duration (Y4795C and R2435L) or higher frequency (R2163H). Notably, myotubes expressing these MH + CCD mutations in RyR1 exhibited both increased [Ca2+]i and reduced sarcoplasmic reticulum (SR) Ca2+ content. We conclude that MH-only mutations modestly increase basal release-channel activity in a manner insufficient to alter net SR Ca2+ content ("compensated leak"), whereas the mixed MH + CCD phenotype arises from mutations that enhance basal activity to a level sufficient to promote SR Ca2+ depletion, elevate [Ca2+]i, and reduce maximal VGCR ("decompensated leak").
Figures
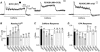

References
-
- Avila, G., E. H. Lee, C. F. Perez, P. D. Allen, and R. T. Dirksen. 2003a. FKBP12 binding to RyR1 modulates excitation-contraction coupling in mouse skeletal myotubes. J. Biol. Chem. 278:22600–22608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

