Cholesterol depletion increases membrane stiffness of aortic endothelial cells
- PMID: 15347591
- PMCID: PMC1304801
- DOI: 10.1529/biophysj.104.040634
Cholesterol depletion increases membrane stiffness of aortic endothelial cells
Abstract
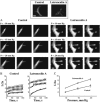
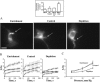
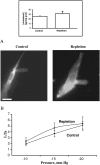
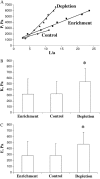
This study has investigated the effect of cellular cholesterol on membrane deformability of bovine aortic endothelial cells. Cellular cholesterol content was depleted by exposing the cells to methyl-beta-cyclodextrin or enriched by exposing the cells to methyl-beta-cyclodextrin saturated with cholesterol. Control cells were treated with methyl-beta-cyclodextrin-cholesterol at a molar ratio that had no effect on the level of cellular cholesterol. Mechanical properties of the cells with different cholesterol contents were compared by measuring the degree of membrane deformation in response to a step in negative pressure applied to the membrane by a micropipette. The experiments were performed on substrate-attached cells that maintained normal morphology. The data were analyzed using a standard linear elastic half-space model to calculate Young elastic modulus. Our observations show that, in contrast to the known effect of cholesterol on membrane stiffness of lipid bilayers, cholesterol depletion of bovine aortic endothelial cells resulted in a significant decrease in membrane deformability and a corresponding increase in the value of the elastic coefficient of the membrane, indicating that cholesterol-depleted cells are stiffer than control cells. Repleting the cells with cholesterol reversed the effect. An increase in cellular cholesterol to a level higher than that of normal cells, however, had no effect on the elastic properties of bovine aortic endothelial cells. We also show that although cholesterol depletion had no apparent effect on the intensity of F-actin-specific fluorescence, disrupting F-actin with latrunculin A abrogated the stiffening effect. We suggest that cholesterol depletion increases the stiffness of the membrane by altering the properties of the submembrane F-actin and/or its attachment to the membrane.
Figures


References
-
- Brener, E., S. Rubinstein, G. Cohen, K. Shternall, J. Rivlin, and H. Breitbart. 2003. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 68:837–845. - PubMed
-
- Brown, A. D., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221–17224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

