Mobile actin clusters and traveling waves in cells recovering from actin depolymerization
- PMID: 15347592
- PMCID: PMC1304815
- DOI: 10.1529/biophysj.104.047589
Mobile actin clusters and traveling waves in cells recovering from actin depolymerization
Abstract
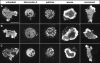
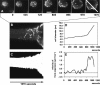
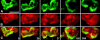
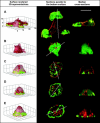
At the leading edge of a motile cell, actin polymerizes in close apposition to the plasma membrane. Here we ask how the machinery for force generation at a leading edge is established de novo after the global depolymerization of actin. The depolymerization is accomplished by latrunculin A, and the reorganization of actin upon removal of the drug is visualized in Dictyostelium cells by total internal reflection fluorescence microscopy. The actin filament system is reorganized in three steps. First, F-actin assembles into globular complexes that move along the bottom surface of the cells at velocities up to 10 microm/min. These clusters are transient structures that eventually disassemble, fuse, or divide. In a second step, clusters merge into a contiguous zone at the cell border that spreads and gives rise to actin waves traveling on a planar membrane. Finally, normal cell shape and motility are resumed. These data show that the initiation of actin polymerization is separated in Dictyostelium from front protrusion, and that the coupling of polymerization to protrusion is a later step in the reconstitution of a leading edge.
Figures






References
-
- Axelrod, D. 2003. Total internal reflection fluorescence microscopy in cell biology. In Methods in Enzymology, Vol. 361, Biophotonics Part B. G. Marriott and I. Parker, editors. Academic Press, New York. 1–33. - PubMed
-
- Bretschneider, T., S. Diez, K. Anderson, J. Heuser, M. Clarke, A. Müller-Taubenberger, J. Köhler, and G. Gerisch. 2004. Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14:1–10. - PubMed
-
- Bretschneider, T., J. Jonkman, J. Köhler, O. Medalia, K. Barisic, I. Weber, E. H. K. Stelzer, W. Baumeister, and G. Gerisch. 2002. Dynamic organization of the actin system in the motile cells of Dictyostelium. J. Muscle Res. Cell Motil. 23:639–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

