The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes
- PMID: 15347783
- PMCID: PMC523334
- DOI: 10.1104/pp.104.047977
The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes
Erratum in
- Plant Physiol. 2004 Oct;136(2):3409
Abstract
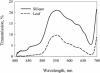
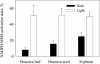
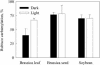
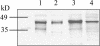
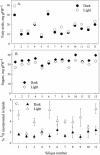
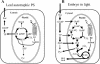
Seeds of many plant species are green during embryogenesis. To directly assess the influence of light on the physiological status of green oilseeds in planta, Brassica napus and soybean (Glycine max) seeds were rapidly dissected from plants growing in the light or dark. The activation state of malate dehydrogenase, which reflects reduced thioredoxin and NADP/NADPH ratios, was found to be as high in seeds exposed to light as in leaves and to decrease in the dark. Rubisco was highly activated (carbamylated) in both light and dark, most likely reflecting high seed CO(2) concentrations. Activities of Rubisco and phosphoribulokinase were sufficient to account for significant refixation of CO(2) produced during B. napus oil biosynthesis. To determine the influence of light on oil synthesis in planta, siliques on intact plants in full sunlight or detached siliques fed (3)H(2)O were partly covered with aluminum foil. Seeds from light and dark sections were analyzed, and fatty acid accumulation was found to be higher in seeds exposed to light than seeds from dark sections. The spectrum of light filtering through silique walls and the pigment composition of developing B. napus embryos were determined. In addition to a low chlorophyll a/b ratio, the carotenoid pigments of seeds can provide additional capture of the green light that filters through siliques. Together, these results demonstrate that even the low level of light reaching seeds plays a substantial role in activating light-regulated enzymes, increasing fatty acid synthesis, and potentially powering refixation of CO(2).
Figures
Similar articles
-
Computational analysis of storage synthesis in developing Brassica napus L. (oilseed rape) embryos: flux variability analysis in relation to ¹³C metabolic flux analysis.Plant J. 2011 Aug;67(3):513-25. doi: 10.1111/j.1365-313X.2011.04611.x. Epub 2011 May 31. Plant J. 2011. PMID: 21501261
-
Plant biochemistry: green catalytic converter.Nature. 2004 Dec 9;432(7018):684. doi: 10.1038/432684b. Nature. 2004. PMID: 15592396 No abstract available.
-
Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds.Nature. 2004 Dec 9;432(7018):779-82. doi: 10.1038/nature03145. Nature. 2004. PMID: 15592419
-
Fatty acid synthesis: from CO2 to functional genomics.Biochem Soc Trans. 2000 Dec;28(6):567-73. Biochem Soc Trans. 2000. PMID: 11171129 Review.
-
Chloroembryos: a unique photosynthesis system.J Plant Physiol. 2013 Sep 1;170(13):1131-8. doi: 10.1016/j.jplph.2013.04.011. Epub 2013 May 24. J Plant Physiol. 2013. PMID: 23706538 Review.
Cited by
-
Storage reserve accumulation in Arabidopsis: metabolic and developmental control of seed filling.Arabidopsis Book. 2008;6:e0113. doi: 10.1199/tab.0113. Epub 2008 Jul 24. Arabidopsis Book. 2008. PMID: 22303238 Free PMC article.
-
Plasticity and constraints on fatty acid composition in the phospholipids and triacylglycerols of Arabidopsis accessions grown at different temperatures.BMC Plant Biol. 2013 Apr 17;13:63. doi: 10.1186/1471-2229-13-63. BMC Plant Biol. 2013. PMID: 23594395 Free PMC article.
-
The role of pheophorbide a oxygenase expression and activity in the canola green seed problem.Plant Physiol. 2006 Sep;142(1):88-97. doi: 10.1104/pp.106.084483. Epub 2006 Jul 14. Plant Physiol. 2006. PMID: 16844830 Free PMC article.
-
Dynamic Metabolic Profiles and Tissue-Specific Source Effects on the Metabolome of Developing Seeds of Brassica napus.PLoS One. 2015 Apr 28;10(4):e0124794. doi: 10.1371/journal.pone.0124794. eCollection 2015. PLoS One. 2015. PMID: 25919591 Free PMC article.
-
Carbon and nitrogen provisions alter the metabolic flux in developing soybean embryos.Plant Physiol. 2013 Mar;161(3):1458-75. doi: 10.1104/pp.112.203299. Epub 2013 Jan 11. Plant Physiol. 2013. PMID: 23314943 Free PMC article.
References
-
- Aach H, Heise KP (1997) On the compartmentation of triacylglycerol synthesis in developing seeds of Brassica napus. Bot Acta 111: 123–129
-
- Asokanthan PS, Johnson RW, Griffith M, Krol M (1997) The photosynthetic potential of canola embryos. Physiol Plant 101: 353–360
-
- Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152: 141–145 - PubMed
-
- Browse J, Slack CR (1985) Fatty-acid synthesis in plastids from maturing safflower and linseed cotyledons. Planta 166: 74–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

