Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance
- PMID: 15347789
- PMCID: PMC523346
- DOI: 10.1104/pp.104.045187
Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance
Abstract
Salinity is one of the major factors that limits geographical distribution of plants and adversely affects crop productivity and quality. We report here high-level expression of betaine aldehyde dehydrogenase (BADH) in cultured cells, roots, and leaves of carrot (Daucus carota) via plastid genetic engineering. Homoplasmic transgenic plants exhibiting high levels of salt tolerance were regenerated from bombarded cell cultures via somatic embryogenesis. Transformation efficiency of carrot somatic embryos was very high, with one transgenic event per approximately seven bombarded plates under optimal conditions. In vitro transgenic carrot cells transformed with the badh transgene were visually green in color when compared to untransformed carrot cells, and this offered a visual selection for transgenic lines. BADH enzyme activity was enhanced 8-fold in transgenic carrot cell cultures, grew 7-fold more, and accumulated 50- to 54-fold more betaine (93-101 micromol g(-1) dry weight of beta-Ala betaine and Gly betaine) than untransformed cells grown in liquid medium containing 100 mm NaCl. Transgenic carrot plants expressing BADH grew in the presence of high concentrations of NaCl (up to 400 mm), the highest level of salt tolerance reported so far among genetically modified crop plants. BADH expression was 74.8% in non-green edible parts (carrots) containing chromoplasts, and 53% in proplastids of cultured cells when compared to chloroplasts (100%) in leaves. Demonstration of plastid transformation via somatic embryogenesis utilizing non-green tissues as recipients of foreign DNA for the first time overcomes two of the major obstacles in extending this technology to important crop plants.
Figures
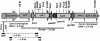

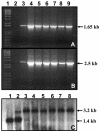
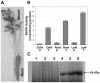
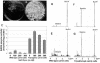

References
-
- Alia, Kondo Y, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen THH, Murata N (1999) Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol 40: 279–288 - PubMed
-
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem-2—inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 - PubMed
-
- Bessieres MA, Gibon Y, Lefeuvre JC, Larher L (1999) A single-step purification for glycine betaine determination in plant extracts by isocratic HPLC. J Agric Food Chem 47: 3718–3722 - PubMed
-
- Bogorad L (2000) Engineering chloroplasts: an alternative site for foreign genes, proteins, reactions and products. Trends Biotechnol 18: 257–263 - PubMed
-
- Cushman JC, Bohnert HJ (2000) Genomic approaches to plant stress tolerance. Curr Opin Plant Biol 3: 117–124 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

