Genetic elucidation of nitric oxide signaling in incompatible plant-pathogen interactions
- PMID: 15347797
- PMCID: PMC523349
- DOI: 10.1104/pp.104.042499
Genetic elucidation of nitric oxide signaling in incompatible plant-pathogen interactions
Abstract
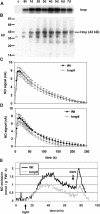
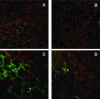
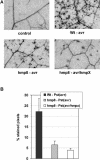
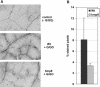
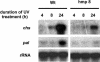
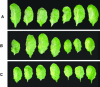
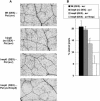
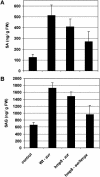
Recent experiments indicate that nitric oxide (NO) plays a pivotal role in disease resistance and several other physiological processes in plants. However, most of the current information about the function of NO in plants is based on pharmacological studies, and additional approaches are therefore required to ascertain the role of NO as an important signaling molecule in plants. We have expressed a bacterial nitric oxide dioxygenase (NOD) in Arabidopsis plants and/or avirulent Pseudomonas syringae pv tomato to study incompatible plant-pathogen interactions impaired in NO signaling. NOD expression in transgenic Arabidopsis resulted in decreased NO levels in planta and attenuated a pathogen-induced NO burst. Moreover, NOD expression in plant cells had very similar effects on plant defenses compared to NOD expression in avirulent Pseudomonas. The defense responses most affected by NO reduction during the incompatible interaction were decreased H(2)O(2) levels during the oxidative burst and a blockage of Phe ammonia lyase expression, the key enzyme in the general phenylpropanoid pathway. Expression of the NOD furthermore blocked UV light-induced Phe ammonia lyase and chalcone synthase gene expression, indicating a general signaling function of NO in the activation of the phenylpropanoid pathway. NO possibly functions in incompatible plant-pathogen interactions by inhibiting the plant antioxidative machinery, and thereby ensuring locally prolonged H(2)O(2) levels. Additionally, albeit to a lesser extent, we observed decreases in salicylic acid production, a diminished development of hypersensitive cell death, and a delay in pathogenesis-related protein 1 expression during these NO-deficient plant-pathogen interactions. Therefore, this genetic approach confirms that NO is an important regulatory component in the signaling network of plant defense responses.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

