AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta
- PMID: 15347798
- PMCID: PMC523317
- DOI: 10.1104/pp.104.042234
AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta
Abstract
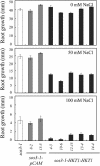
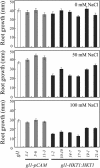
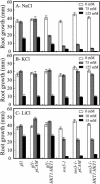
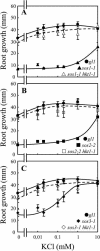
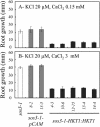
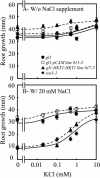
Genetic and physiological data establish that Arabidopsis AtHKT1 facilitates Na(+) homeostasis in planta and by this function modulates K(+) nutrient status. Mutations that disrupt AtHKT1 function suppress NaCl sensitivity of sos1-1 and sos2-2, as well as of sos3-1 seedlings grown in vitro and plants grown in controlled environmental conditions. hkt1 suppression of sos3-1 NaCl sensitivity is linked to higher Na(+) content in the shoot and lower content of the ion in the root, reducing the Na(+) imbalance between these organs that is caused by sos3-1. AtHKT1 transgene expression, driven by its innate promoter, increases NaCl but not LiCl or KCl sensitivity of wild-type (Col-0 gl1) or of sos3-1 seedlings. NaCl sensitivity induced by AtHKT1 transgene expression is linked to a lower K(+) to Na(+) ratio in the root. However, hkt1 mutations increase NaCl sensitivity of both seedlings in vitro and plants grown in controlled environmental conditions, which is correlated with a lower K(+) to Na(+) ratio in the shoot. These results establish that AtHKT1 is a focal determinant of Na(+) homeostasis in planta, as either positive or negative modulation of its function disturbs ion status that is manifested as salt sensitivity. K(+)-deficient growth of sos1-1, sos2-2, and sos3-1 seedlings is suppressed completely by hkt1-1. AtHKT1 transgene expression exacerbates K(+) deficiency of sos3-1 or wild-type seedlings. Together, these results indicate that AtHKT1 controls Na(+) homeostasis in planta and through this function regulates K(+) nutrient status.
Figures





References
-
- Amtmann A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. Adv Bot Res 29: 75–112
-
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285: 1256–1258 - PubMed
-
- Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12: 431–434 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

