Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors
- PMID: 15347806
- PMCID: PMC518807
- DOI: 10.1073/pnas.0405310101
Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors
Abstract
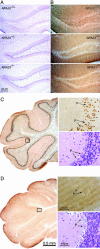
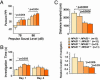
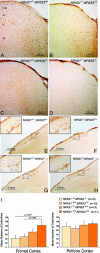
Laboratory mice bearing inactivating mutations in the genes encoding the NPAS1 and NPAS3 transcription factors have been shown to exhibit a spectrum of behavioral and neurochemical abnormalities. Behavioral abnormalities included diminished startle response, as measured by prepulse inhibition, and impaired social recognition. NPAS1/NPAS3-deficient mice also exhibited stereotypic darting behavior at weaning and increased locomotor activity. Immunohistochemical staining assays showed that the NPAS1 and NPAS3 proteins are expressed in inhibitory interneurons and that the viability and anatomical distribution of these neurons are unaffected by the absence of either transcription factor. Adult brain tissues from NPAS3- and NPAS1/NPAS3-deficient mice exhibited a distinct reduction in reelin, a large, secreted protein whose expression has been reported to be attenuated in the postmortem brain tissue of patients with schizophrenia. These observations raise the possibility that a regulatory program controlled in inhibitory interneurons by the NPAS1 and NPAS3 transcription factors may be either substantively or tangentially relevant to psychosis.
Figures

References
-
- Brunskill, E. W., Witte, D. P., Shreiner, A. B. & Potter, S. S. (1999) Mech. Dev. 88, 237–241. - PubMed
-
- Dudley, C. A., Erbel-Sieler, C., Estill, S. J., Reick, M., Franken, P., Pitts, S. & McKnight, S. L. (2003) Science 301, 379–383. - PubMed
-
- Rutter, J., Reick, M., Wu, L. C. & McKnight, S. L. (2001) Science 293, 510–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

