Role of KaiC phosphorylation in the circadian clock system of Synechococcus elongatus PCC 7942
- PMID: 15347812
- PMCID: PMC518855
- DOI: 10.1073/pnas.0403906101
Role of KaiC phosphorylation in the circadian clock system of Synechococcus elongatus PCC 7942
Abstract
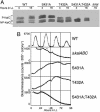
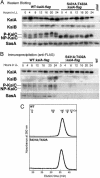
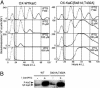
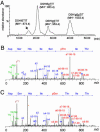
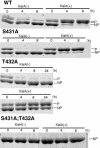
In the cyanobacterium Synechococcus elongatus PCC 7942, KaiA, KaiB, and KaiC are essential proteins for the generation of a circadian rhythm. KaiC is proposed as a negative regulator of the circadian expression of all genes in the genome, and its phosphorylation is regulated positively by KaiA and negatively by KaiB and shows a circadian rhythm in vivo. To study the functions of KaiC phosphorylation in the circadian clock system, we identified two autophosphorylation sites, Ser-431 and Thr-432, by using mass spectrometry (MS). We generated Synechococcus mutants in which these residues were substituted for alanine by using site-directed mutagenesis. Phosphorylation of KaiC was reduced in the single mutants and was completely abolished in the double mutant, indicating that KaiC is also phosphorylated at these sites in vivo. These mutants lost circadian rhythm, indicating that phosphorylation at each of the two sites is essential for the control of the circadian oscillation. Although the nonphosphorylatable mutant KaiC was able to form a hexamer in vitro, it failed to form a clock protein complex with KaiA, KaiB, and SasA in the Synechococcus cells. When nonphosphorylatable KaiC was overexpressed, the kaiBC promoter activity was only transiently repressed. These results suggest that KaiC phosphorylation regulates its transcriptional repression activity by controlling its binding affinity for other clock proteins.
Figures
Comment in
-
Meshing the gears of the cyanobacterial circadian clock.Proc Natl Acad Sci U S A. 2004 Sep 21;101(38):13697-8. doi: 10.1073/pnas.0405623101. Epub 2004 Sep 14. Proc Natl Acad Sci U S A. 2004. PMID: 15367731 Free PMC article. No abstract available.
References
-
- Bunning, E. (1973) The Physiological Clock (Springer, New York), 3rd Ed.
-
- Golden, S. S., Ishiura, M., Johnson, C. H. & Kondo, T. (1997) Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 327–354. - PubMed
-
- Iwasaki, H. & Kondo, T. (2000) Plant Cell Physiol. 41, 1013–1020. - PubMed
-
- Ishiura, M., Kutsuna, S., Aoki, S., Iwasaki, H., Andersson, C. R., Tanabe, A., Golden, S. S., Johnson, C. H. & Kondo, T. (1998) Science 281, 1519–1523. - PubMed
-
- Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

