Ku80 is required but not sufficient for Galpha13-mediated endodermal differentiation in P19 embryonic carcinoma cells
- PMID: 15351736
- PMCID: PMC5998665
- DOI: 10.1016/j.bbrc.2004.08.092
Ku80 is required but not sufficient for Galpha13-mediated endodermal differentiation in P19 embryonic carcinoma cells
Abstract
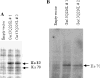
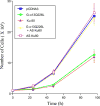
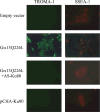
We have shown that a constitutively active Galpha13 (Galpha13Q226L) induces differentiation in P19 embryonic carcinoma cells to an endodermal phenotype. In this report, we demonstrate that Ku, a heterodimer of p80 (Ku80) and p70 (Ku70), is upregulated in P19 cells overexpressing Galpha13Q226L. Ku is the regulatory subunit of the DNA-dependent protein kinase and is primarily involved in DNA repair and recombination. Ku80 also is a somatostatin receptor. We show that while overexpression of Ku80 drastically reduced P19 cell proliferation, it was not sufficient to induce endodermal differentiation. However, coexpression of Galpha13Q226L and an antisense Ku80 abrogated the retarded growth rate and endodermal differentiation observed in cells expressing only Galpha13Q226L. Overexpression of Galpha13Q226L or Ku80 downregulated RNA polymerase I-mediated transcriptional activity and overexpression of antisense Ku80 restored the activity to control level. These results suggest that Ku80 is required for Galpha13-mediated endodermal differentiation in P19 cells.
Copyright 2004 Elsevier Inc.
Figures

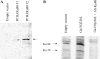
References
-
- Mimori T, Hardin JA, Steitz JA. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986;261:2274–2278. - PubMed
-
- Le Romancer ML, Reyl-Desmars F, Cherifi Y, Pigeon C, Bottari S, Meyer O, Lewin MJM. The 86-kDa subunit of autoantigen Ku is a somatostatin receptor regulating protein phosphatase-2A activity. J Biol Chem. 1994;269:7464–17468. - PubMed
-
- Scheer U, Zentgraf H, pug A. In: The Cell Nucleus II. Busch H, Rothblum L, editors. Academic Press; New York: 1982. pp. 143–176.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

