Antigen-independent acquisition of MHC class II molecules by human T lymphocytes
- PMID: 15351785
- PMCID: PMC1986719
- DOI: 10.1093/intimm/dxh154
Antigen-independent acquisition of MHC class II molecules by human T lymphocytes
Abstract
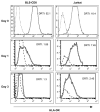
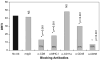
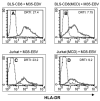
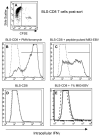
We report here that human T lymphocytes have the capacity of acquiring large amounts of MHC class II molecules from various types of antigen-presenting cells (APC) in an antigen-independent manner. The transfer of MHC class II molecules from APC to T cell required direct cell-to-cell contact and appeared to involve the interaction of numerous adhesion molecules between these cells. Depletion of cholesterol from the plasma membrane reduced the amount of MHC class II transferred onto the T cells. Most significantly, the newly acquired MHC class II molecules were capable of efficiently presenting antigen to T helper cells. These results suggest that T cells are able to interact with other T cells to regulate immune responses by presenting MHC peptide complexes that have been snatched away from nearby APC.
Figures





Similar articles
-
Class II MHC/peptide complexes are released from APC and are acquired by T cell responders during specific antigen recognition.J Immunol. 1999 Nov 15;163(10):5201-10. J Immunol. 1999. PMID: 10553040
-
Presentation of acquired peptide-MHC class II ligands by CD4+ regulatory T cells or helper cells differentially regulates antigen-specific CD4+ T cell response.J Immunol. 2011 Feb 15;186(4):2148-55. doi: 10.4049/jimmunol.1002917. Epub 2011 Jan 17. J Immunol. 2011. PMID: 21242518
-
Peptide-specific intercellular transfer of MHC class II to CD4+ T cells directly from the immunological synapse upon cellular dissociation.J Immunol. 2005 Jan 1;174(1):80-9. doi: 10.4049/jimmunol.174.1.80. J Immunol. 2005. PMID: 15611230
-
The ins and outs of MHC class II-mediated antigen processing and presentation.Nat Rev Immunol. 2015 Apr;15(4):203-16. doi: 10.1038/nri3818. Epub 2015 Feb 27. Nat Rev Immunol. 2015. PMID: 25720354 Free PMC article. Review.
-
T cells as antigen-presenting cells.Immunol Today. 1994 Jul;15(7):312-5. doi: 10.1016/0167-5699(94)90078-7. Immunol Today. 1994. PMID: 7522009 Review.
Cited by
-
Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses.J Immunol. 2007 Sep 1;179(5):2731-40. doi: 10.4049/jimmunol.179.5.2731. J Immunol. 2007. PMID: 17709486 Free PMC article.
-
Cells resident to precision templated 40-µm pore scaffolds generate small extracellular vesicles that affect CD4+ T cell phenotypes through regulatory TLR4 signaling.Acta Biomater. 2023 Aug;166:119-132. doi: 10.1016/j.actbio.2023.05.007. Epub 2023 May 6. Acta Biomater. 2023. PMID: 37150279 Free PMC article.
-
Induction of a Regulatory Phenotype in CD3+ CD4+ HLA-DR+ T Cells after Allogeneic Mixed Lymphocyte Culture; Indications of Both Contact-Dependent and -Independent Activation.Int J Mol Sci. 2017 Jul 24;18(7):1603. doi: 10.3390/ijms18071603. Int J Mol Sci. 2017. PMID: 28737722 Free PMC article.
-
CD4(+) T cell-released exosomes inhibit CD8(+) cytotoxic T-lymphocyte responses and antitumor immunity.Cell Mol Immunol. 2011 Jan;8(1):23-30. doi: 10.1038/cmi.2010.59. Epub 2010 Dec 13. Cell Mol Immunol. 2011. PMID: 21200381 Free PMC article.
-
The effect of edible bird's nests on the expression of MHC-II and costimulatory molecules of C57BL/6 mouse splenocytes.Biochem Biophys Rep. 2023 Aug 25;35:101534. doi: 10.1016/j.bbrep.2023.101534. eCollection 2023 Sep. Biochem Biophys Rep. 2023. PMID: 37671389 Free PMC article.
References
-
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259. - PubMed
-
- McDevitt HO. Discovering the role of the major histocompatibility complex in the immune response. Annu Rev Immunol. 2000;18:1. - PubMed
-
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881. - PubMed
-
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474. - PubMed
-
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

