NAD+ surfaces again
- PMID: 15352307
- PMCID: PMC1133982
- DOI: 10.1042/BJ20041217
NAD+ surfaces again
Abstract
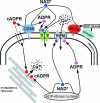
NAD+ and its metabolites serve important functions in intracellular signalling. NAD+-mediated regulatory processes also take place on the cell surface, particularly of immune cells. In this issue of the Biochemical Journal, Gerth et al. have demonstrated a new mechanism of Ca2+ uptake into monocytes which is triggered by NAD+ or its degradation product, ADP-ribose. These observations point to a hitherto unknown Ca2+-influx mechanism and underscore the potential significance of NAD+ and ADP-ribose as signalling molecules on the extracellular side of the plasma membrane.
Figures
Comment on
-
Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes.Biochem J. 2004 Sep 15;382(Pt 3):849-56. doi: 10.1042/BJ20040979. Biochem J. 2004. PMID: 15233622 Free PMC article.
Similar articles
-
Extracellular NAD+ regulates intracellular free calcium concentration in human monocytes.Biochem J. 2004 Sep 15;382(Pt 3):849-56. doi: 10.1042/BJ20040979. Biochem J. 2004. PMID: 15233622 Free PMC article.
-
[ADP-ribose and cADP-ribose--endogenous regulators of cellular ionic balance. Cardiotropic effects of ADP-ribose].Usp Fiziol Nauk. 2006 Jan-Mar;37(1):3-17. Usp Fiziol Nauk. 2006. PMID: 16522000 Review. Russian.
-
Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system.Ann N Y Acad Sci. 2004 Dec;1028:176-91. doi: 10.1196/annals.1322.021. Ann N Y Acad Sci. 2004. PMID: 15650244 Review.
-
Cyclic ADP-ribose as a universal calcium signal molecule in the nervous system.Neurochem Int. 2007 Jul-Sep;51(2-4):192-9. doi: 10.1016/j.neuint.2007.06.023. Epub 2007 Jun 28. Neurochem Int. 2007. PMID: 17664018 Review.
-
Poly(ADP-ribose) signal in seizures-induced neuron death.Med Hypotheses. 2008 Aug;71(2):283-5. doi: 10.1016/j.mehy.2008.02.012. Epub 2008 Apr 15. Med Hypotheses. 2008. PMID: 18417297
Cited by
-
A lectin receptor kinase as a potential sensor for extracellular nicotinamide adenine dinucleotide in Arabidopsis thaliana.Elife. 2017 Jul 19;6:e25474. doi: 10.7554/eLife.25474. Elife. 2017. PMID: 28722654 Free PMC article.
-
Maintenance of NAD+ Homeostasis in Skeletal Muscle during Aging and Exercise.Cells. 2022 Feb 17;11(4):710. doi: 10.3390/cells11040710. Cells. 2022. PMID: 35203360 Free PMC article. Review.
-
ESEM evaluations of muscle/nanoparticles interface in a rat model.J Mater Sci Mater Med. 2008 Apr;19(4):1515-22. doi: 10.1007/s10856-008-3385-6. Epub 2008 Feb 12. J Mater Sci Mater Med. 2008. PMID: 18266087
-
CD38 expression, function, and gene resequencing in a human lymphoblastoid cell line-based model system.Leuk Lymphoma. 2010 Jul;51(7):1315-25. doi: 10.3109/10428194.2010.483299. Leuk Lymphoma. 2010. PMID: 20470215 Free PMC article.
-
The essential schistosome tegumental ectoenzyme SmNPP5 can block NAD-induced T cell apoptosis.Virulence. 2020 Dec;11(1):568-579. doi: 10.1080/21505594.2020.1770481. Virulence. 2020. PMID: 32441549 Free PMC article.
References
-
- Berger F., Ramírez-Hernández M. H., Ziegler M. The new life of a centenarian: signalling functions of NAD(P) Trends Biochem. Sci. 2004;29:111–118. - PubMed
-
- Okazaki I. J., Moss J. Glycosylphosphatidylinositol-anchored and secretory isoforms of mono-ADP-ribosyltransferases. J. Biol. Chem. 1998;273:23617–23620. - PubMed
-
- Funaro A., Reinis M., Trubiani O., Santi S., Di Primio R., Malavasi F. CD38 functions are regulated through an internalization step. J. Immunol. 1998;160:2238–2247. - PubMed
-
- Zocchi E., Usai C., Guida L., Franco L., Bruzzone S., Passalacqua M., De Flora A. Ligand-induced internalization of CD38 results in intracellular Ca2+ mobilization: role of NAD+ transport across cell membranes. FASEB J. 1999;13:273–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

