Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling
- PMID: 15353546
- PMCID: PMC2172129
- DOI: 10.1083/jcb.200405061
Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling
Abstract
Yeast cells can initiate bud formation at the G1/S transition in a cue-independent manner. Here, we investigate the dynamic nature of the polar cap and the regulation of the GTPase Cdc42 in the establishment of cell polarity. Using analysis of fluorescence recovery after photobleaching, we found that Cdc42 exchanged rapidly between the polar caps and cytosol and that this rapid exchange required its GTPase cycle. A previously proposed positive feedback loop involving actomyosin-based transport of the Cdc42 GTPase is required for the generation of robust cell polarity during bud formation in yeast. Inhibition of actin-based transport resulted in unstable Cdc42 polar caps. Unstable polarity was also observed in mutants lacking Bem1, a protein previously implicated in a feedback loop for Cdc42 activation through a signaling pathway. When Bem1 and actin were both inhibited, polarization completely failed. These results suggest that cell polarity is established through coupling of transport and signaling pathways and maintained actively by balance of flux.
Figures



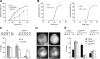
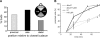


References
-
- Amon, A., S. Irniger, and K. Nasmyth. 1994. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell. 77:1037–1050. - PubMed
-
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

