A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells
- PMID: 15353550
- PMCID: PMC2172116
- DOI: 10.1083/jcb.200403010
A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells
Abstract
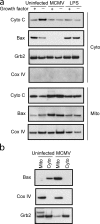
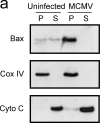
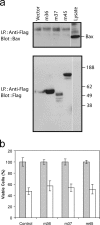
Infection with murine cytomegalovirus (MCMV) has contributed to understanding many aspects of human infection and, additionally, has provided important insight to understanding complex cellular responses. Dendritic cells (DCs) are a major target for MCMV infection. Here, we analyze the effects of MCMV infection on DC viability, and show that infected DCs become resistant to apoptosis induced by growth factor deprivation. The precise contribution of changes in the expression of Bcl-2 family proteins has been assessed and a new checkpoint in the apoptotic pathway identified. Despite their resistance to apoptosis, MCMV-infected DCs showed Bax to be tightly associated with mitochondria and, together with Bak, forming high molecular weight oligomers, changes normally associated with apoptotic cell death. Exposure of a constitutively occluded Bax NH2-terminal epitope was blocked after infection. These results suggest that MCMV has evolved a novel strategy for inhibiting apoptosis and provide evidence that apoptosis can be regulated after translocation, integration, and oligomerization of Bax at the mitochondrial membrane.
Figures



Similar articles
-
Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release.Cancer Res. 2003 Apr 1;63(7):1712-21. Cancer Res. 2003. PMID: 12670926
-
Regulation of the mitochondrial checkpoint in p53-mediated apoptosis confers resistance to cell death.Oncogene. 2002 Jan 24;21(5):748-60. doi: 10.1038/sj.onc.1205125. Oncogene. 2002. PMID: 11850803
-
Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway.EMBO J. 2003 Jul 15;22(14):3580-90. doi: 10.1093/emboj/cdg343. EMBO J. 2003. PMID: 12853473 Free PMC article.
-
vMIA, a viral inhibitor of apoptosis targeting mitochondria.Biochimie. 2002 Feb-Mar;84(2-3):177-85. doi: 10.1016/s0300-9084(02)01367-6. Biochimie. 2002. PMID: 12022948 Review.
-
The proto-oncogene Bcl-2 and its role in regulating apoptosis.Nat Med. 1997 Jun;3(6):614-20. doi: 10.1038/nm0697-614. Nat Med. 1997. PMID: 9176486 Review. No abstract available.
Cited by
-
Prevention of cellular suicide by cytomegaloviruses.Viruses. 2012 Oct 2;4(10):1928-49. doi: 10.3390/v4101928. Viruses. 2012. PMID: 23202447 Free PMC article. Review.
-
Lymphoma cell apoptosis in the liver induced by distant murine cytomegalovirus infection.J Virol. 2006 May;80(10):4801-19. doi: 10.1128/JVI.80.10.4801-4819.2006. J Virol. 2006. PMID: 16641273 Free PMC article.
-
BH3 Profiling Reveals Selectivity by Herpesviruses for Specific Bcl-2 Proteins To Mediate Survival of Latently Infected Cells.J Virol. 2015 May;89(10):5739-46. doi: 10.1128/JVI.00236-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740993 Free PMC article.
-
Induction of apoptosis limits cytomegalovirus cross-species infection.EMBO J. 2006 Jun 7;25(11):2634-42. doi: 10.1038/sj.emboj.7601133. Epub 2006 May 11. EMBO J. 2006. PMID: 16688216 Free PMC article.
-
Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis.J Virol. 2005 Oct;79(19):12205-17. doi: 10.1128/JVI.79.19.12205-12217.2005. J Virol. 2005. PMID: 16160147 Free PMC article.
References
-
- Andrews, D.M., C.E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M.A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077–1084. - PubMed
-
- Antonsson, B., S. Montessuit, B. Sanchez, and J.C. Martinou. 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 276:11615–11623. - PubMed
-
- Ardeshna, K.M., A.R. Pizzey, S. Devereaux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-kappa B signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 96:1039–1046. - PubMed
-
- Arnoult, D., L.M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P.U. Park, J. Sharpe, R.J. Youle, and V.S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA. 101:7988–7993. - PMC - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.T. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

