Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins
- PMID: 15353562
- PMCID: PMC515256
- DOI: 10.1128/MMBR.68.3.373-402.2004
Binary bacterial toxins: biochemistry, biology, and applications of common Clostridium and Bacillus proteins
Abstract
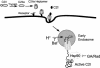
Certain pathogenic species of Bacillus and Clostridium have developed unique methods for intoxicating cells that employ the classic enzymatic "A-B" paradigm for protein toxins. The binary toxins produced by B. anthracis, B. cereus, C. botulinum, C. difficile, C. perfringens, and C. spiroforme consist of components not physically associated in solution that are linked to various diseases in humans, animals, or insects. The "B" components are synthesized as precursors that are subsequently activated by serine-type proteases on the targeted cell surface and/or in solution. Following release of a 20-kDa N-terminal peptide, the activated "B" components form homoheptameric rings that subsequently dock with an "A" component(s) on the cell surface. By following an acidified endosomal route and translocation into the cytosol, "A" molecules disable a cell (and host organism) via disruption of the actin cytoskeleton, increasing intracellular levels of cyclic AMP, or inactivation of signaling pathways linked to mitogen-activated protein kinase kinases. Recently, B. anthracis has gleaned much notoriety as a biowarfare/bioterrorism agent, and of primary interest has been the edema and lethal toxins, their role in anthrax, as well as the development of efficacious vaccines and therapeutics targeting these virulence factors and ultimately B. anthracis. This review comprehensively surveys the literature and discusses the similarities, as well as distinct differences, between each Clostridium and Bacillus binary toxin in terms of their biochemistry, biology, genetics, structure, and applications in science and medicine. The information may foster future studies that aid novel vaccine and drug development, as well as a better understanding of a conserved intoxication process utilized by various gram-positive, spore-forming bacteria.
Figures





References
-
- Acheson, D. W. K., and G. T. Keusch. 1999. The family of Shiga toxins, p. 229-242. In J. E. Alouf and J. H. Freer (ed.), The comprehensive sourcebook of bacterial protein toxins. Academic Press, Inc., New York, N.Y.
-
- Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. - PubMed
-
- Ahuja, N., P. Kumar, S. Alam, M. Gupta, and R. Bhatnagar. 2003. Deletion mutants of protective antigen that inhibit anthrax toxin both in vitro and in vivo. Biochem. Biophys. Res. Commun. 307:446-450. - PubMed
-
- Ahuja, N., P. Kumar, and R. Bhatnagar. 2001. Hydrophobic residues Phe552, Phe554, Ile562, Leu566, and Ile574 are required for oligomerization of anthrax protective antigen. Biochem. Biophys. Res. Commun. 287:542-549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

