CO-sensing mechanisms
- PMID: 15353565
- PMCID: PMC515253
- DOI: 10.1128/MMBR.68.3.453-473.2004
CO-sensing mechanisms
Abstract
Carbon monoxide (CO) has long been known to have dramatic physiological effects on organisms ranging from bacteria to humans, but recently there have a number of suggestions that organisms might have specific sensors for CO. This article reviews the current evidence for a variety of proteins with demonstrated or potential CO-sensing ability. Particular emphasis is placed on the molecular description of CooA, a heme-containing CO sensor from Rhodospirillum rubrum, since its biological role as a CO sensor is clear and we have substantial insight into the basis of its sensing ability.
Figures
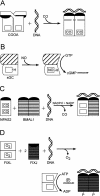

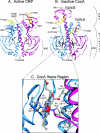

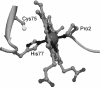

References
-
- Adams, M. W. W., and E. I. Stiefel. 2000. Organometallic iron: the key to biological hydrogen metabolism. Curr. Opin. Chem. Biol. 4:214-220. - PubMed
-
- Akimoto, S., A. Tanaka, K. Nakamura, Y. Shiro, and H. Nakamura. 2003. O2-specific regulation of the ferrous heme-based sensor kinase FixL from Sinorhizobium meliloti and its aberrant inactivation in the ferric form. Biochem. Biophys. Res. Commun. 304:136-142. - PubMed
-
- Albracht, S. P. J., and R. Hedderich. 2000. Learning from hydrogenases: location of a proton pump and of a second FMN in bovine NADH-ubiquinone oxidoreductase (complex I). FEBS Lett. 485:1-6. - PubMed
-
- Aono, S. 2003. Biochemical and biophysical properties of the CO-sensing transcriptional activator CooA. Acc. Chem. Res. 36:825-831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

