Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion
- PMID: 15353570
- PMCID: PMC515249
- DOI: 10.1128/MMBR.68.3.560-602.2004
Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion
Abstract
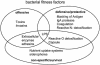
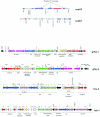
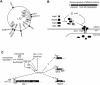
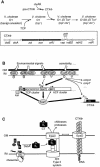
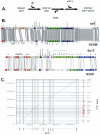
Comparative genomics demonstrated that the chromosomes from bacteria and their viruses (bacteriophages) are coevolving. This process is most evident for bacterial pathogens where the majority contain prophages or phage remnants integrated into the bacterial DNA. Many prophages from bacterial pathogens encode virulence factors. Two situations can be distinguished: Vibrio cholerae, Shiga toxin-producing Escherichia coli, Corynebacterium diphtheriae, and Clostridium botulinum depend on a specific prophage-encoded toxin for causing a specific disease, whereas Staphylococcus aureus, Streptococcus pyogenes, and Salmonella enterica serovar Typhimurium harbor a multitude of prophages and each phage-encoded virulence or fitness factor makes an incremental contribution to the fitness of the lysogen. These prophages behave like "swarms" of related prophages. Prophage diversification seems to be fueled by the frequent transfer of phage material by recombination with superinfecting phages, resident prophages, or occasional acquisition of other mobile DNA elements or bacterial chromosomal genes. Prophages also contribute to the diversification of the bacterial genome architecture. In many cases, they actually represent a large fraction of the strain-specific DNA sequences. In addition, they can serve as anchoring points for genome inversions. The current review presents the available genomics and biological data on prophages from bacterial pathogens in an evolutionary framework.
Figures






References
-
- Affolter, M., C. Parent-Vaugeois, and A. Anderson. 1983. Curing and induction of the Fels 1 and Fels 2 prophages in the Ames mutagen tester strains of Salmonella typhimurium. Mutat. Res. 110:243-262. - PubMed
-
- Allison, G. E., D. C. Angeles, P. Huan, and N. K. Verma. 2003. Morphology of temperate bacteriophage SfV and characterisation of the DNA packaging and capsid genes: the structural genes evolved from two different phage families. Virology 308:114-127. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

