Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors
- PMID: 15353585
- PMCID: PMC518787
- DOI: 10.1073/pnas.0405361101
Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors
Abstract
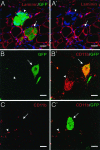
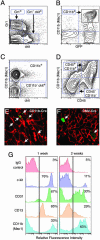
Adult bone marrow-derived cells can participate in muscle regeneration after bone marrow transplantation. In recent studies a single hematopoietic stem cell (HSC) was shown to give rise to cells that not only reconstituted all of the lineages of the blood, but also contributed to mature muscle fibers. However, the relevant HSC derivative with this potential has not yet been definitively identified. Here we use fluorescence-activated cell sorter-based protocols to test distinct hematopoietic fractions and show that only fractions containing c-kit(+) immature myelomonocytic precursors are capable of contributing to muscle fibers after i.m. injection. Although these cells belong to the myeloid lineage, they do not include mature CD11b(+) myelomonocytic cells, such as macrophages. Of the four sources of mature macrophages tested that were derived either from monocytic culture, bone marrow, peripheral blood after granulocyte colony-stimulating factor mobilization, or injured muscle, none contributed to muscle. In addition, after transplantation of bone marrow isolated from CD11b-Cre-transgenic mice into the Cre-reporter strain (Z/EG), no GFP myofibers were detected, demonstrating that macrophages expressing CD11b do not fuse with myofibers. Irrespective of the underlying mechanisms, these data suggest that the HSC derivatives that integrate into regenerating muscle fibers exist in the pool of hematopoietic cells known as myelomonocytic progenitors.
Figures

Similar articles
-
Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates.Nat Med. 2003 Dec;9(12):1520-7. doi: 10.1038/nm963. Epub 2003 Nov 16. Nat Med. 2003. PMID: 14625546
-
Contribution of hematopoietic stem cells to skeletal muscle.Nat Med. 2003 Dec;9(12):1528-32. doi: 10.1038/nm959. Epub 2003 Nov 16. Nat Med. 2003. PMID: 14625543
-
Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells.Stem Cells. 2004;22(7):1292-304. doi: 10.1634/stemcells.2004-0090. Stem Cells. 2004. PMID: 15579647
-
Stem cell plasticity in muscle and bone marrow.Ann N Y Acad Sci. 2001 Jun;938:208-18; discussion 218-20. doi: 10.1111/j.1749-6632.2001.tb03591.x. Ann N Y Acad Sci. 2001. PMID: 11458510 Review.
-
Fidelity and infidelity in commitment to B-lymphocyte lineage development.Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
Cited by
-
Pharmacological manipulation of the RAR/RXR signaling pathway maintains the repopulating capacity of hematopoietic stem cells in culture.Mol Endocrinol. 2009 Feb;23(2):188-201. doi: 10.1210/me.2008-0121. Epub 2008 Dec 23. Mol Endocrinol. 2009. PMID: 19106195 Free PMC article.
-
Senolytic effects of exercise in human muscles require acute inflammation.Aging (Albany NY). 2024 May 15;16(10):8599-8610. doi: 10.18632/aging.205827. Epub 2024 May 15. Aging (Albany NY). 2024. PMID: 38752873 Free PMC article. Clinical Trial.
-
Net39 protects muscle nuclei from mechanical stress during the pathogenesis of Emery-Dreifuss muscular dystrophy.J Clin Invest. 2023 Jul 3;133(13):e163333. doi: 10.1172/JCI163333. J Clin Invest. 2023. PMID: 37395273 Free PMC article.
-
A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches.Differentiation. 2009 Sep-Oct;78(2-3):185-94. doi: 10.1016/j.diff.2009.08.004. Differentiation. 2009. PMID: 19751902 Free PMC article. Review.
-
Satellite cells and the muscle stem cell niche.Physiol Rev. 2013 Jan;93(1):23-67. doi: 10.1152/physrev.00043.2011. Physiol Rev. 2013. PMID: 23303905 Free PMC article. Review.
References
-
- Grounds, M. D., White, J. D., Rosenthal, N. & Bogoyevitch, M. A. (2002) J. Histochem. Cytochem. 50, 589–610. - PubMed
-
- Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. & Sharkis, S. J. (2001) Cell 105, 369–377. - PubMed
-
- Lagasse, E., Connors, H., Al-Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I. L. & Grompe, M. (2000) Nat. Med. 6, 1229–1234. - PubMed
-
- Bittner, R. E., Schofer, C., Weipoltshammer, K., Ivanova, S., Streubel, B., Hauser, E., Freilinger, M., Hoger, H., Elbe-Burger, A. & Wachtler, F. (1999) Anat. Embryol. 199, 391–396. - PubMed
-
- Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. (2000) Science 290, 1779–1782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

