Different functional roles of T1R subunits in the heteromeric taste receptors
- PMID: 15353592
- PMCID: PMC521102
- DOI: 10.1073/pnas.0404384101
Different functional roles of T1R subunits in the heteromeric taste receptors
Abstract
The T1R receptors, a family of taste-specific class C G protein-coupled receptors, mediate mammalian sweet and umami tastes. The structure-function relationships of T1R receptors remain largely unknown. In this study, we demonstrate the different functional roles of T1R extracellular and transmembrane domains in ligand recognition and G protein coupling. Similar to other family C G protein-coupled receptors, the N-terminal Venus flytrap domain of T1R2 is required for recognizing sweeteners, such as aspartame and neotame. The G protein coupling requires the transmembrane domain of T1R2. Surprisingly, the C-terminal transmembrane domain of T1R3 is required for recognizing sweetener cyclamate and sweet taste inhibitor lactisole. Because T1R3 is the common subunit in the sweet taste receptor and the umami taste receptor, we tested the interaction of lactisole and cyclamate with the umami taste receptor. Lactisole inhibits the activity of the human T1R1/T1R3 receptor, and, as predicted, blocked the umami taste of l-glutamate in human taste tests. Cyclamate does not activate the T1R1/T1R3 receptor by itself, but potentiates the receptor's response to l-glutamate. Taken together, these findings demonstrate the different functional roles of T1R3 and T1R2 and the presence of multiple ligand binding sites on the sweet taste receptor.
Figures
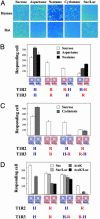

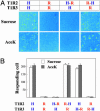


Comment in
-
Unraveling the biochemistry of sweet and umami tastes.Proc Natl Acad Sci U S A. 2004 Sep 28;101(39):13972-3. doi: 10.1073/pnas.0405991101. Epub 2004 Sep 21. Proc Natl Acad Sci U S A. 2004. PMID: 15383662 Free PMC article. No abstract available.
Similar articles
-
Modulation of sweet taste by umami compounds via sweet taste receptor subunit hT1R2.PLoS One. 2015 Apr 8;10(4):e0124030. doi: 10.1371/journal.pone.0124030. eCollection 2015. PLoS One. 2015. PMID: 25853419 Free PMC article.
-
The liaison of sweet and savory.Chem Senses. 2006 Mar;31(3):221-5. doi: 10.1093/chemse/bjj022. Epub 2005 Dec 29. Chem Senses. 2006. PMID: 16384922 Clinical Trial.
-
The heterodimeric sweet taste receptor has multiple potential ligand binding sites.Curr Pharm Des. 2006;12(35):4591-600. doi: 10.2174/138161206779010350. Curr Pharm Des. 2006. PMID: 17168764 Review.
-
Detection of sweet and umami taste in the absence of taste receptor T1r3.Science. 2003 Aug 8;301(5634):850-3. doi: 10.1126/science.1087155. Epub 2003 Jul 17. Science. 2003. PMID: 12869700
-
Taste information derived from T1R-expressing taste cells in mice.Biochem J. 2016 Mar 1;473(5):525-36. doi: 10.1042/BJ20151015. Biochem J. 2016. PMID: 26912569 Review.
Cited by
-
Molecular neurobiology of Drosophila taste.Curr Opin Neurobiol. 2015 Oct;34:140-8. doi: 10.1016/j.conb.2015.06.001. Epub 2015 Jun 21. Curr Opin Neurobiol. 2015. PMID: 26102453 Free PMC article. Review.
-
Temperature-dependent conformational change affecting Tyr11 and sweetness loops of brazzein.Proteins. 2013 Jun;81(6):919-25. doi: 10.1002/prot.24259. Epub 2013 Feb 25. Proteins. 2013. PMID: 23349025 Free PMC article.
-
An efficient Escherichia coli expression system for the production of a functional N-terminal domain of the T1R3 taste receptor.Bioengineered. 2013 Jan-Feb;4(1):25-9. doi: 10.4161/bioe.21877. Epub 2012 Aug 22. Bioengineered. 2013. PMID: 22909933 Free PMC article.
-
Molecular mechanisms of taste transduction in vertebrates.Odontology. 2009 Jan;97(1):1-7. doi: 10.1007/s10266-008-0095-y. Epub 2009 Jan 29. Odontology. 2009. PMID: 19184292 Review.
-
GPCRs and Signal Transducers: Interaction Stoichiometry.Trends Pharmacol Sci. 2018 Jul;39(7):672-684. doi: 10.1016/j.tips.2018.04.002. Epub 2018 May 5. Trends Pharmacol Sci. 2018. PMID: 29739625 Free PMC article. Review.
References
-
- Hoon, M. A., Adler, E., Lindemeier, J., Battey, J. F., Ryba, N. J. & Zuker, C. S. (1999) Cell 96, 541–551. - PubMed
-
- Montmayeur, J. P., Liberles, S. D., Matsunami, H. & Buck, L. B. (2001) Nat. Neurosci. 4, 492–498. - PubMed
-
- Max, M., Shanker, Y. G., Huang, L., Rong, M., Liu, Z., Campagne, F., Weinstein, H., Damak, S. & Margolskee, R. F. (2001) Nat. Genet. 28, 58–63. - PubMed
-
- Kitagawa, M., Kusakabe, Y., Miura, H., Ninomiya, Y. & Hino, A. (2001) Biochem. Biophys. Res. Commun. 283, 236–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

