A nonpolio enterovirus with respiratory tropism causes poliomyelitis in intercellular adhesion molecule 1 transgenic mice
- PMID: 15353596
- PMCID: PMC518806
- DOI: 10.1073/pnas.0403998101
A nonpolio enterovirus with respiratory tropism causes poliomyelitis in intercellular adhesion molecule 1 transgenic mice
Abstract
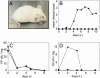
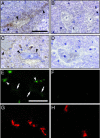
Coxsackievirus A21 (CAV21) is classified within the species Human enterovirus C (HEV-C) of the Enterovirus genus of picornaviruses. HEV-C share striking homology with the polioviruses (PV), their closest kin among the enteroviruses. Despite a high level of sequence identity, CAV21 and PV cause distinct clinical disease typically attributed to their differential use of host receptors. PV cause poliomyelitis, whereas CAV21 shares a receptor and a propensity to cause upper respiratory tract infections with the major group rhinoviruses. As a model for CAV21 infection, we have developed transgenic mice that express human intercellular adhesion molecule 1, the cell-surface receptor for CAV21. Surprisingly, CAV21 administered to these mice via the intramuscular route causes a paralytic condition consistent with poliomyelitis. The virus appears to invade the CNS by retrograde axonal transport, as has been demonstrated to occur in analogous PV infections. We detected human intercellular adhesion molecule 1 expression on both transgenic mouse and human spinal cord anterior horn motor neurons, indicating that members of HEV-C may share PV's potential to elicit poliomyelitis in humans.
Figures




References
-
- Grist, N. R., Bell, E. J. & Assaad, A. (1978) Prog. Med. Virol. 24, 114–157. - PubMed
-
- Dalldorf, G. & Melnick, J. L. (1965) in Viral and Rickettsial Infections of Man, eds. Horsfall, F. L & Tamm, I. (Lippincott, Philadelphia), 4th Ed., pp. 474–512.
-
- King, A. M. Q., Brown, F., Christian, P., Hovi, T., Hyypia, T., Knowles, N. J., Lemon, S. M., Minor, P. D., Palmenberg, A. C., Skern, T., et al. (2000) in Virus Taxonomy, Seventh Report of the International Committee on Taxonomy of Viruses, eds. Van Regenmortel, M. H. V., Fanquet, C. M., Bishop, D. H. L., Carstens, E. B., Estes, M. K., Lemon, S. M., Maniloff, J., Mayo, M. A., McGeoch, D. J., Pringle, C. R., et al. (Academic, San Diego), pp. 657–678.
-
- Pulli, T., Koskimies, P. & Hyypia, T. (1995) Virology 212, 30–38. - PubMed
-
- Pöyry, T., Kinnunen, L., Hyypiä, T., Brown, B., Horsnell, C., Hovi, T. & Stanway, G. (1996) J. Gen. Virol. 77, 1699–1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

