Multiple activities of the human splicing factor ASF
- PMID: 1535526
- PMCID: PMC6057360
Multiple activities of the human splicing factor ASF
Abstract
The effects of human alternative splicing factor, ASF, on in vitro splicing of adenovirus E1A pre-mRNA were examined. E1A pre-mRNA is a complex substrate, and splicing in HeLa cell nuclear extracts produces six different RNAs using three alternative 5' splice sites and two 3' splice sites. Addition of excess ASF to splicing reactions produced a simplified splicing pattern, in which only one spliced product, 13S RNA, was detected. Inhibition of 12S and 9S splicing, which use 5' splice sites upstream of the 13S 5' splice site, extends previous observations that when multiple 5' splice sites compete for the same 3' splice site, ASF causes preferential selection of the proximal 5' splice site. However, inhibition of the other splices, which use a different upstream 3' splice site, represents a novel activity of ASF, as competition between 5' splice sites is not involved. The effect of ASF on 12S splicing was found to depend on its position relative to competing 5' splice sites, indicating that the ability of ASF to activate proximal 5' splice sites is position- but not sequence-dependent. Finally, addition of small amounts of ASF to ASF-lacking S100 extract was able to activate distal as well as proximal 5' splice sites in two of three pre-mRNAs tested, indicating that in these cases changes in the concentration of ASF alone can be sufficient to modulate alternative 5' splice site selection.
Figures


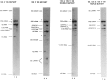

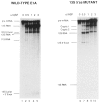
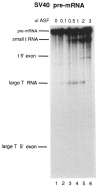
Similar articles
-
The second RNA-binding domain of the human splicing factor ASF/SF2 is the critical domain controlling adenovirus E1A alternative 5'-splice site selection.Biochem J. 2004 Jul 15;381(Pt 2):343-50. doi: 10.1042/BJ20040408. Biochem J. 2004. PMID: 15068396 Free PMC article.
-
Exonic splicing enhancer-dependent selection of the bovine papillomavirus type 1 nucleotide 3225 3' splice site can be rescued in a cell lacking splicing factor ASF/SF2 through activation of the phosphatidylinositol 3-kinase/Akt pathway.J Virol. 2003 Feb;77(3):2105-15. doi: 10.1128/jvi.77.3.2105-2115.2003. J Virol. 2003. PMID: 12525645 Free PMC article.
-
A novel protein factor is required for use of distal alternative 5' splice sites in vitro.Mol Cell Biol. 1991 Dec;11(12):5945-53. doi: 10.1128/mcb.11.12.5945-5953.1991. Mol Cell Biol. 1991. PMID: 1658620 Free PMC article.
-
ASF/SF2: a splice site selector.Trends Biochem Sci. 1991 Dec;16(12):452-3. doi: 10.1016/0968-0004(91)90176-v. Trends Biochem Sci. 1991. PMID: 1781021 Review. No abstract available.
-
SR proteins and splicing control.Genes Dev. 1996 Jul 1;10(13):1569-79. doi: 10.1101/gad.10.13.1569. Genes Dev. 1996. PMID: 8682289 Review. No abstract available.
Cited by
-
The A1 and A1B proteins of heterogeneous nuclear ribonucleoparticles modulate 5' splice site selection in vivo.Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6924-8. doi: 10.1073/pnas.91.15.6924. Proc Natl Acad Sci U S A. 1994. PMID: 8041722 Free PMC article.
-
Overexpression of essential splicing factor ASF/SF2 blocks the temporal shift in adenovirus pre-mRNA splicing and reduces virus progeny formation.J Virol. 2000 Oct;74(19):9002-9. doi: 10.1128/jvi.74.19.9002-9009.2000. J Virol. 2000. PMID: 10982344 Free PMC article.
-
Mammalian protein factors involved in nuclear pre-mRNA splicing.Mol Biol Rep. 1993 Aug;18(2):93-8. doi: 10.1007/BF00986762. Mol Biol Rep. 1993. PMID: 8232300 Review. No abstract available.
-
The protein kinase Clk/Sty directly modulates SR protein activity: both hyper- and hypophosphorylation inhibit splicing.Mol Cell Biol. 1999 Oct;19(10):6991-7000. doi: 10.1128/MCB.19.10.6991. Mol Cell Biol. 1999. PMID: 10490636 Free PMC article.
-
Genetic analysis of the SR protein ASF/SF2: interchangeability of RS domains and negative control of splicing.Genes Dev. 1998 Jul 15;12(14):2222-33. doi: 10.1101/gad.12.14.2222. Genes Dev. 1998. PMID: 9679066 Free PMC article.
References
-
- Amrein H., Gorman M., and Nothiger R. (1988), Cell 55, 1025–1035. - PubMed
-
- Baker B. S. (1989), Nature 340, 521–524. - PubMed
-
- Berk A. J. and Sharp P. A. (1978), Cell 14, 695–711. - PubMed
-
- Bell L. R., Maine E. M., Schedl P., and Cline T. W. (1988), Cell 55, 1037–1046. - PubMed
-
- Bingham P. M., Chou T.-B., Mims I., and Zachar Z. (1988), Trends Genet 4, 134–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
