Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials
- PMID: 15355866
- PMCID: PMC4242214
- DOI: 10.1093/aob/mch193
Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials
Abstract
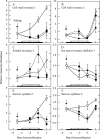
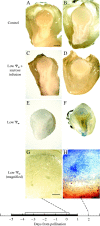
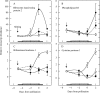
Background and aims: Ovary abortion can occur in maize (Zea mays) if water deficits lower the water potential (psiw) sufficiently to inhibit photosynthesis around the time of pollination. The abortion decreases kernel number. The present work explored the activity of ovary acid invertases and their genes, together with other genes for sucrose-processing enzymes, when this kind of abortion occurred. Cytological evidence suggested that senescence may have been initiated after 2 or 3 d of low psiw, and the expression of some likely senescence genes was also determined.
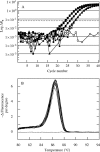
Methods: Ovary abortion was assessed at kernel maturity. Acid invertase activities were localized in vivo and in situ. Time courses for mRNA abundance were measured with real time PCR. Sucrose was fed to the stems to vary the sugar flux.
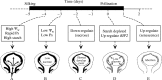
Key results: Many kernels developed in controls but most aborted when psiw became low. Ovary invertase was active in controls but severely inhibited at low psiw for cell wall-bound forms in vivo and soluble forms in situ. All ovary genes for sucrose processing enzymes were rapidly down-regulated at low psiw except for a gene for invertase inhibitor peptide that appeared to be constitutively expressed. Some ovary genes for senescence were subsequently up-regulated (RIP2 and PLD1). In some genes, these regulatory changes were reversed by feeding sucrose to the stems. Abortion was partially prevented by feeding sucrose.
Conclusions: A general response to low psiw in maize ovaries was an early down-regulation of genes for sucrose processing enzymes followed by up-regulation of some genes involved in senescence. Because some of these genes were sucrose responsive, the partial prevention of abortion with sucrose feeding may have been caused in part by the differential sugar-responsiveness of these genes. The late up-regulation of senescence genes may have caused the irreversibility of abortion.
Figures
Similar articles
-
Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems.Ann Bot. 2004 Jul;94(1):75-86. doi: 10.1093/aob/mch123. Epub 2004 May 24. Ann Bot. 2004. PMID: 15159218 Free PMC article.
-
Starch and the control of kernel number in maize at low water potentials.Plant Physiol. 1999 Sep;121(1):25-36. doi: 10.1104/pp.121.1.25. Plant Physiol. 1999. PMID: 10482657 Free PMC article.
-
Imaging and quantifying carbohydrate transport to the developing ovaries of maize.Ann Bot. 2005 Oct;96(5):939-49. doi: 10.1093/aob/mci246. Epub 2005 Aug 12. Ann Bot. 2005. PMID: 16100223 Free PMC article.
-
Grain yields with limited water.J Exp Bot. 2004 Nov;55(407):2385-94. doi: 10.1093/jxb/erh219. Epub 2004 Jul 30. J Exp Bot. 2004. PMID: 15286147 Review.
-
Functional reversion to identify controlling genes in multigenic responses: analysis of floral abortion.J Exp Bot. 2007;58(2):267-77. doi: 10.1093/jxb/erl177. Epub 2006 Nov 14. J Exp Bot. 2007. PMID: 17105969 Review.
Cited by
-
Deficit irrigation and sustainable water-resource strategies in agriculture for China's food security.J Exp Bot. 2015 Apr;66(8):2253-69. doi: 10.1093/jxb/erv034. Epub 2015 Apr 3. J Exp Bot. 2015. PMID: 25873664 Free PMC article. Review.
-
CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis.BMC Plant Biol. 2018 Oct 11;18(1):228. doi: 10.1186/s12870-018-1456-5. BMC Plant Biol. 2018. PMID: 30309330 Free PMC article.
-
Physio-morphological traits and osmoregulation strategies of hybrid maize (Zea mays) at the seedling stage in response to water-deficit stress.Protoplasma. 2022 Jul;259(4):869-883. doi: 10.1007/s00709-021-01707-0. Epub 2021 Sep 28. Protoplasma. 2022. PMID: 34581924
-
Transcriptomic and metabolomics responses to elevated cell wall invertase activity during tomato fruit set.J Exp Bot. 2017 Jul 10;68(15):4263-4279. doi: 10.1093/jxb/erx219. J Exp Bot. 2017. PMID: 28922759 Free PMC article.
-
Differential Role for Trehalose Metabolism in Salt-Stressed Maize.Plant Physiol. 2015 Oct;169(2):1072-89. doi: 10.1104/pp.15.00729. Epub 2015 Aug 12. Plant Physiol. 2015. PMID: 26269545 Free PMC article.
References
-
- Allen SM, Helentjaris T, Bate NJ. 2001. Novel invertase inhibitors and methods of use. Pioneer Hi-Bred Inc. (US); E.I. Du Pont De Nemours and Company (US). WO Patent 0158939-A. Date issued: 16 August.
-
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. 1996. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. Journal of Cell Biology 133: 1251–1263. - PMC - PubMed

