Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species
- PMID: 15356189
- PMCID: PMC6729932
- DOI: 10.1523/JNEUROSCI.1899-04.2004
Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species
Abstract
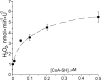
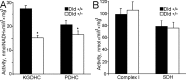
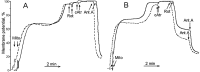
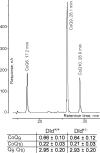
Mitochondria-produced reactive oxygen species (ROS) are thought to contribute to cell death caused by a multitude of pathological conditions. The molecular sites of mitochondrial ROS production are not well established but are generally thought to be located in complex I and complex III of the electron transport chain. We measured H(2)O(2) production, respiration, and NADPH reduction level in rat brain mitochondria oxidizing a variety of respiratory substrates. Under conditions of maximum respiration induced with either ADP or carbonyl cyanide p-trifluoromethoxyphenylhydrazone,alpha-ketoglutarate supported the highest rate of H(2)O(2) production. In the absence of ADP or in the presence of rotenone, H(2)O(2) production rates correlated with the reduction level of mitochondrial NADPH with various substrates, with the exception of alpha-ketoglutarate. Isolated mitochondrial alpha-ketoglutarate dehydrogenase (KGDHC) and pyruvate dehydrogenase (PDHC) complexes produced superoxide and H(2)O(2). NAD(+) inhibited ROS production by the isolated enzymes and by permeabilized mitochondria. We also measured H(2)O(2) production by brain mitochondria isolated from heterozygous knock-out mice deficient in dihydrolipoyl dehydrogenase (Dld). Although this enzyme is a part of both KGDHC and PDHC, there was greater impairment of KGDHC activity in Dld-deficient mitochondria. These mitochondria also produced significantly less H(2)O(2) than mitochondria isolated from their littermate wild-type mice. The data strongly indicate that KGDHC is a primary site of ROS production in normally functioning mitochondria.
Figures


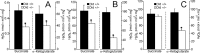
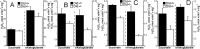
Similar articles
-
Characteristics of alpha-glycerophosphate-evoked H2O2 generation in brain mitochondria.J Neurochem. 2007 Feb;100(3):650-63. doi: 10.1111/j.1471-4159.2006.04223.x. J Neurochem. 2007. PMID: 17263793
-
Topology of superoxide production from different sites in the mitochondrial electron transport chain.J Biol Chem. 2002 Nov 22;277(47):44784-90. doi: 10.1074/jbc.M207217200. Epub 2002 Sep 16. J Biol Chem. 2002. PMID: 12237311
-
Inhibition of the alpha-ketoglutarate dehydrogenase-mediated reactive oxygen species generation by lipoic acid.J Neurochem. 2009 May;109 Suppl 1:222-9. doi: 10.1111/j.1471-4159.2009.05942.x. J Neurochem. 2009. PMID: 19393031
-
Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources.Antioxid Redox Signal. 2005 Sep-Oct;7(9-10):1140-9. doi: 10.1089/ars.2005.7.1140. Antioxid Redox Signal. 2005. PMID: 16115017 Review.
-
Generation of superoxide by the mitochondrial Complex I.Biochim Biophys Acta. 2006 May-Jun;1757(5-6):553-61. doi: 10.1016/j.bbabio.2006.03.013. Epub 2006 Apr 17. Biochim Biophys Acta. 2006. PMID: 16678117 Review.
Cited by
-
Pyruvate Dehydrogenase Kinases in the Nervous System: Their Principal Functions in Neuronal-glial Metabolic Interaction and Neuro-metabolic Disorders.Curr Neuropharmacol. 2012 Dec;10(4):393-403. doi: 10.2174/157015912804143586. Curr Neuropharmacol. 2012. PMID: 23730261 Free PMC article.
-
Estrogen regulation of mitochondrial bioenergetics: implications for prevention of Alzheimer's disease.Adv Pharmacol. 2012;64:327-71. doi: 10.1016/B978-0-12-394816-8.00010-6. Adv Pharmacol. 2012. PMID: 22840752 Free PMC article. Review.
-
Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species.Redox Biol. 2015;4:381-98. doi: 10.1016/j.redox.2015.02.001. Epub 2015 Feb 7. Redox Biol. 2015. PMID: 25744690 Free PMC article. Review.
-
Underlying molecular alterations in human dihydrolipoamide dehydrogenase deficiency revealed by structural analyses of disease-causing enzyme variants.Hum Mol Genet. 2019 Oct 15;28(20):3339-3354. doi: 10.1093/hmg/ddz177. Hum Mol Genet. 2019. PMID: 31334547 Free PMC article.
-
Mitochondrial-Derived Peptides in Diabetes and Its Complications.Front Endocrinol (Lausanne). 2022 Feb 3;12:808120. doi: 10.3389/fendo.2021.808120. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35185787 Free PMC article. Review.
References
-
- Arrigoni O, Singer TP (1962) Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nauchni Tr Vissh Med Inst Sofiia 193: 1256-1258. - PubMed
-
- Azzi A, Montecucco C, Richter C (1975) The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun 65: 597-603. - PubMed
-
- Barja G (1999) Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr 31: 347-366. - PubMed
-
- Bunik VI (2003) 2-Oxo acid dehydrogenase complexes in redox regulation. Eur J Biochem 270: 1036-1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
