Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons
- PMID: 15356191
- PMCID: PMC1890032
- DOI: 10.1523/JNEUROSCI.1941-04.2004
Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons
Abstract
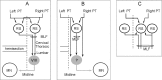
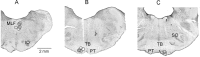
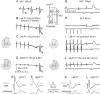
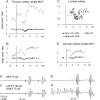
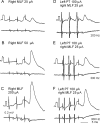
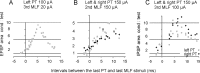
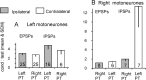
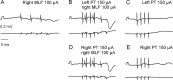
Contralateral pyramidal tract (PT) neurons arising in the primary motor cortex are the major route through which volitional limb movements are controlled. However, the contralateral hemiparesis that follows PT neuron injury on one side may be counteracted by ipsilateral of actions of PT neurons from the undamaged side. To investigate the spinal relays through which PT neurons may influence ipsilateral motoneurons, we analyzed the synaptic actions evoked by stimulation of the ipsilateral pyramid on hindlimb motoneurons after transecting the descending fibers of the contralateral PT at a low thoracic level. The results show that ipsilateral PT neurons can affect limb motoneurons trisynaptically by activating contralaterally descending reticulospinal neurons, which in turn activate spinal commissural interneurons that project back across to motoneurons ipsilateral to the stimulated pyramidal tract. Stimulation of the pyramids alone did not evoke synaptic actions in motoneurons but potently facilitated disynaptic EPSPs and IPSPs evoked by stimulation of reticulospinal tract fibers in the medial longitudinal fascicle. In parallel with this double-crossed pathway, corticospinal neurons could also evoke ipsilateral actions via ipsilateral descending reticulospinal tract fibers, acting through ipsilaterally located spinal interneurons. Because the actions mediated by commissural interneurons were found to be stronger than those of ipsilateral premotor interneurons, the study leads to the conclusion that ipsilateral actions of corticospinal neurons via commissural interneurons may provide a better opportunity for recovery of function in hemiparesis produced by corticospinal tract injury.
Figures



Similar articles
-
Uncrossed actions of feline corticospinal tract neurones on hindlimb motoneurones evoked via ipsilaterally descending pathways.J Physiol. 2007 Apr 1;580(Pt 1):119-32. doi: 10.1113/jphysiol.2006.122721. Epub 2007 Jan 25. J Physiol. 2007. PMID: 17255171 Free PMC article.
-
How to enhance ipsilateral actions of pyramidal tract neurons.J Neurosci. 2005 Aug 10;25(32):7401-5. doi: 10.1523/JNEUROSCI.1838-05.2005. J Neurosci. 2005. PMID: 16093391 Free PMC article.
-
Ipsilateral actions from the feline red nucleus on hindlimb motoneurones.J Physiol. 2008 Dec 15;586(24):5865-84. doi: 10.1113/jphysiol.2008.163998. Epub 2008 Oct 20. J Physiol. 2008. PMID: 18936076 Free PMC article.
-
How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions.Neuroscientist. 2006 Feb;12(1):67-79. doi: 10.1177/1073858405283392. Neuroscientist. 2006. PMID: 16394194 Free PMC article. Review.
-
Propriospinal transmission of part of the corticospinal excitation in humans.Muscle Nerve. 2002 Aug;26(2):155-72. doi: 10.1002/mus.1240. Muscle Nerve. 2002. PMID: 12210379 Review.
Cited by
-
Neuronal relays in double crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurones.J Physiol. 2006 Sep 1;575(Pt 2):527-41. doi: 10.1113/jphysiol.2006.112425. Epub 2006 Jun 1. J Physiol. 2006. PMID: 16740611 Free PMC article.
-
Ipsilateral and Contralateral Interactions in Spinal Locomotor Circuits Mediated by V1 Neurons: Insights from Computational Modeling.Int J Mol Sci. 2022 May 16;23(10):5541. doi: 10.3390/ijms23105541. Int J Mol Sci. 2022. PMID: 35628347 Free PMC article.
-
Processing information related to centrally initiated locomotor and voluntary movements by feline spinocerebellar neurones.J Physiol. 2011 Dec 1;589(Pt 23):5709-25. doi: 10.1113/jphysiol.2011.213678. Epub 2011 Sep 19. J Physiol. 2011. PMID: 21930605 Free PMC article.
-
Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans.Brain. 2014 May;137(Pt 5):1394-409. doi: 10.1093/brain/awu038. Epub 2014 Apr 8. Brain. 2014. PMID: 24713270 Free PMC article.
-
Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade.J Neurosci. 2008 Jul 16;28(29):7426-34. doi: 10.1523/JNEUROSCI.1078-08.2008. J Neurosci. 2008. PMID: 18632946 Free PMC article.
References
-
- Alstermark B, Pinter M, Sasaki S (1983) Brainstem relay of disynaptic pyramidal EPSPs to neck motoneurons in the cat. Brain Res 259: 147-150. - PubMed
-
- Alstermark B, Lundberg A, Pinter M, Sasaki S (1987) Subpopulations and functions of long C3-C5 propriospinal neurones. Brain Res 404: 395-400. - PubMed
-
- Alstermark B, Pinter MJ, Sasaki S (1992) Descending pathways mediating disynaptic excitation of dorsal neck motoneurones in the cat: brain stem relay. Neurosci Res 15: 42-57. - PubMed
-
- Armand J, Kuypers HG (1980) Cells of origin of crossed and uncrossed corticospinal fibers in the cat: a quantitative horseradish peroxidase study. Exp Brain Res 40: 23-34. - PubMed
-
- Armand J, Holstege G, Kuypers HG (1985) Differential corticospinal projections in the cat. An autoradiographic tracing study. Brain Res 343: 351-355. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
