L1.1 is involved in spinal cord regeneration in adult zebrafish
- PMID: 15356195
- PMCID: PMC6729920
- DOI: 10.1523/JNEUROSCI.2420-04.2004
L1.1 is involved in spinal cord regeneration in adult zebrafish
Abstract
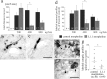
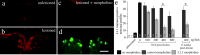
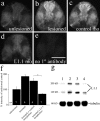
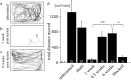
Adult zebrafish, in contrast to mammals, regrow axons descending from the brainstem after spinal cord transection. L1.1, a homolog of the mammalian recognition molecule L1, is upregulated by brainstem neurons during axon regrowth. However, its functional relevance for regeneration is unclear. Here, we show with a novel morpholino-based approach that reducing L1.1 protein expression leads to impaired locomotor recovery as well as reduced regrowth and synapse formation of axons of supraspinal origin after spinal cord transection. This indicates that L1.1 contributes to successful regrowth of axons from the brainstem and locomotor recovery after spinal cord transection in adult zebrafish.
Figures
References
-
- Asher RA, Morgenstern DA, Moon LD, Fawcett JW (2001) Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res 132: 611-619. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME (2004) The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269-277. - PubMed
-
- Becker CG, Schweitzer J, Feldner J, Schachner M, Becker T (2004) Tenascin-R as a repellent guidance molecule for newly growing and regenerating optic axons in adult zebrafish. Mol Cell Neurosci 26: 376-389. - PubMed
-
- Becker T, Becker CG (2001) Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J Comp Neurol 433: 131-147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
