Chloride accumulation in mammalian olfactory sensory neurons
- PMID: 15356206
- PMCID: PMC6729923
- DOI: 10.1523/JNEUROSCI.2115-04.2004
Chloride accumulation in mammalian olfactory sensory neurons
Abstract
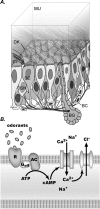
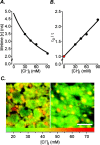
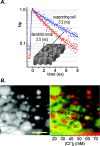
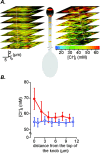
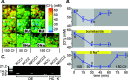
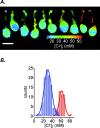
The generation of an excitatory receptor current in mammalian olfactory sensory neurons (OSNs) involves the sequential activation of two distinct types of ion channels: cAMP-gated Ca(2+)-permeable cation channels and Ca(2+)-gated Cl(-) channels, which conduct a depolarizing Cl(-) efflux. This unusual transduction mechanism requires an outward-directed driving force for Cl(-), established by active accumulation of Cl(-) within the lumen of the sensory cilia. We used two-photon fluorescence lifetime imaging microscopy of the Cl(-)-sensitive dye 6-methoxy-quinolyl acetoethyl ester to measure the intracellular Cl(-) concentration in dendritic knobs of OSNs from mice and rats. We found a uniform intracellular Cl(-) concentration in the range of 40-50 mm, which is indicative of active Cl(-) accumulation. Functional assays and PCR experiments revealed that NKCC1-mediated Cl(-) uptake through the apical membrane counteracts Cl(-) depletion in the sensory cilia, and thus maintains the responsiveness of OSNs to odor stimulation. To permit Cl(-) accumulation, OSNs avoid the "chloride switch": they do not express KCC2, the main Cl(-) extrusion cotransporter operating in neurons of the adult CNS. Cl(-) accumulation provides OSNs with the driving force for the depolarizing Cl(-) current that is the basis of the low-noise receptor current in these neurons.
Figures
Similar articles
-
Mechanism of the excitatory Cl- response in mouse olfactory receptor neurons.Neuron. 2005 Feb 17;45(4):553-61. doi: 10.1016/j.neuron.2005.01.012. Neuron. 2005. PMID: 15721241 Free PMC article.
-
Molecular components of signal amplification in olfactory sensory cilia.Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):6052-7. doi: 10.1073/pnas.0909032107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231443 Free PMC article.
-
Trigeminal ganglion neurons of mice show intracellular chloride accumulation and chloride-dependent amplification of capsaicin-induced responses.PLoS One. 2012;7(11):e48005. doi: 10.1371/journal.pone.0048005. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23144843 Free PMC article.
-
The ins and outs of intracellular chloride in olfactory receptor neurons.Neuron. 2005 Feb 17;45(4):481-2. doi: 10.1016/j.neuron.2005.02.002. Neuron. 2005. PMID: 15721233 Review.
-
Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters.Physiol Rev. 2005 Apr;85(2):423-93. doi: 10.1152/physrev.00011.2004. Physiol Rev. 2005. PMID: 15788703 Review.
Cited by
-
Direct interaction of CaVβ with actin up-regulates L-type calcium currents in HL-1 cardiomyocytes.J Biol Chem. 2015 Feb 20;290(8):4561-4572. doi: 10.1074/jbc.M114.573956. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533460 Free PMC article.
-
Smelling better with chloride.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11063-11065. doi: 10.1073/pnas.1614328113. Epub 2016 Sep 22. Proc Natl Acad Sci U S A. 2016. PMID: 27660238 Free PMC article. No abstract available.
-
Paradoxical electro-olfactogram responses in TMEM16B knock-out mice.Chem Senses. 2023 Jan 1;48:bjad003. doi: 10.1093/chemse/bjad003. Chem Senses. 2023. PMID: 36744918 Free PMC article.
-
Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia.Curr Top Med Chem. 2005;5(6):547-55. doi: 10.2174/1568026054367629. Curr Top Med Chem. 2005. PMID: 16022677 Free PMC article. Review.
-
Spatial and temporal distribution patterns of Na-K-2Cl cotransporter in adult and developing mouse retinas.Vis Neurosci. 2008 Mar-Apr;25(2):109-23. doi: 10.1017/S0952523808080164. Vis Neurosci. 2008. PMID: 18442435 Free PMC article.
References
-
- Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R (2001) Immunolocalization of the Na+-K+-2Cl- cotransporter in peripheral nervous tissue of vertebrates. Neuroscience 104: 569-582. - PubMed
-
- Buck LB (2000) The molecular architecture of odor and pheromone sensing in mammals. Cell 100: 611-618. - PubMed
-
- Denk W, Svoboda K (1997) Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18: 351-357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
