Attentional modulation of motion integration of individual neurons in the middle temporal visual area
- PMID: 15356211
- PMCID: PMC6729935
- DOI: 10.1523/JNEUROSCI.5102-03.2004
Attentional modulation of motion integration of individual neurons in the middle temporal visual area
Abstract
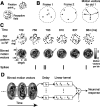
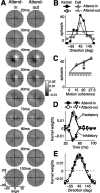
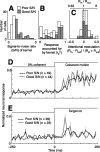
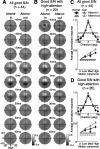
We examined how spatially directed attention affected the integration of motion in neurons of the middle temporal (MT) area of visual cortex. We recorded from single MT neurons while monkeys performed a motion detection task under two attentional states. Using 0% coherent random dot motion, we estimated the optimal linear transfer function (or kernel) between the global motion and the neuronal response. This linear kernel filtered the random dot motion across direction, speed, and time. Slightly less than one-half of the neurons produced reasonably well defined kernels that also tended to account for both the directional selectivity and responses to coherent motion of different strengths. This subpopulation of cells had faster, more transient, and more robust responses to visual stimuli than neurons with kernels that did not contain well defined regions of integration. For those neurons that had large attentional modulation and produced well defined kernels, we found attention scaled the temporal profile of the transfer function with no appreciable shift in time or change in shape. Thus, for MT neurons described by a linear transfer function, attention produced a multiplicative scaling of the temporal integration window.
Figures





Similar articles
-
Attentional modulation of behavioral performance and neuronal responses in middle temporal and ventral intraparietal areas of macaque monkey.J Neurosci. 2002 Mar 1;22(5):1994-2004. doi: 10.1523/JNEUROSCI.22-05-01994.2002. J Neurosci. 2002. PMID: 11880530 Free PMC article.
-
Directional signals in the prefrontal cortex and in area MT during a working memory for visual motion task.J Neurosci. 2006 Nov 8;26(45):11726-42. doi: 10.1523/JNEUROSCI.3420-06.2006. J Neurosci. 2006. PMID: 17093094 Free PMC article.
-
Feature-based attention increases the selectivity of population responses in primate visual cortex.Curr Biol. 2004 May 4;14(9):744-51. doi: 10.1016/j.cub.2004.04.028. Curr Biol. 2004. PMID: 15120065
-
Response latencies of neurons in visual areas MT and MST of monkeys with striate cortex lesions.Neuropsychologia. 2003;41(13):1738-56. doi: 10.1016/s0028-3932(03)00176-3. Neuropsychologia. 2003. PMID: 14527538
-
A geometric view on early and middle level visual coding.Spat Vis. 2000;13(2-3):193-9. doi: 10.1163/156856800741207. Spat Vis. 2000. PMID: 11198231 Review.
Cited by
-
Attention influences single unit and local field potential response latencies in visual cortical area V4.J Neurosci. 2012 Nov 7;32(45):16040-50. doi: 10.1523/JNEUROSCI.0489-12.2012. J Neurosci. 2012. PMID: 23136440 Free PMC article.
-
The construction of confidence in a perceptual decision.Front Integr Neurosci. 2012 Sep 21;6:79. doi: 10.3389/fnint.2012.00079. eCollection 2012. Front Integr Neurosci. 2012. PMID: 23049504 Free PMC article.
-
Attention modulates the responses of simple cells in monkey primary visual cortex.J Neurosci. 2005 Nov 23;25(47):11023-33. doi: 10.1523/JNEUROSCI.2904-05.2005. J Neurosci. 2005. PMID: 16306415 Free PMC article.
-
Spatiotemporal dynamics of feature-based attention spread: evidence from combined electroencephalographic and magnetoencephalographic recordings.J Neurosci. 2012 Jul 11;32(28):9671-6. doi: 10.1523/JNEUROSCI.0439-12.2012. J Neurosci. 2012. PMID: 22787052 Free PMC article.
-
Spatial attention and the latency of neuronal responses in macaque area V4.J Neurosci. 2007 Sep 5;27(36):9632-7. doi: 10.1523/JNEUROSCI.2734-07.2007. J Neurosci. 2007. PMID: 17804623 Free PMC article.
References
-
- Albrecht DG, Geisler WS (1991) Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci 7: 531-546. - PubMed
-
- Albrecht DG, Hamilton DB (1982) Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48: 217-237. - PubMed
-
- Andersen RA, Snyder LH, Bradley DC, Xing J (1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303-330. - PubMed
-
- Bair W, Cavanaugh JR, Movshon JA (1997) Reconstructing stimulus velocity from neuronal responses in area MT. In: Advances in neural information processing systems (Mozer MC, Jordan MI, Petsche T, eds). Cambridge, MA: MIT.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
