Formation of nuclear splicing factor compartments is independent of lamins A/C
- PMID: 15356259
- PMCID: PMC524741
- DOI: 10.1091/mbc.e04-07-0645
Formation of nuclear splicing factor compartments is independent of lamins A/C
Abstract
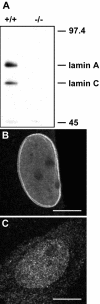
Nuclear lamins are major architectural elements of the mammalian cell nucleus, and they have been implicated in the functional organization of the nuclear interior, possibly by providing structural support for nuclear compartments. Colocalization studies have suggested a structural role for lamins in the formation and maintenance of pre-mRNA splicing factor compartments. Here, we have directly tested this hypothesis by analysis of embryonic fibroblasts from knock-out mice lacking A- and C-type lamins. We show that the morphology and cellular properties of splicing factor compartments are independent of A- and C-type lamins. Genetic loss of lamins A/C has no effect on the cellular distribution of several pre-mRNA splicing factors and does not affect the compartment morphology as examined by light and electron microscopy. The association of splicing factors with the nuclear matrix fraction persists in the absence of lamins A/C. Live cell microscopy demonstrates that the intranuclear positional stability of splicing factor compartments is maintained and that the exchange dynamics of SF2/ASF between the compartments and the nucleoplasm is not affected by loss of lamin A/C. Our results demonstrate that formation and maintenance of intranuclear splicing factor compartments is independent of lamins A/C, and they argue against an essential structural role of lamins A/C in splicing factor compartment morphology.
Figures

Similar articles
-
Colocalization of intranuclear lamin foci with RNA splicing factors.J Cell Sci. 1999 Dec;112 ( Pt 24):4651-61. doi: 10.1242/jcs.112.24.4651. J Cell Sci. 1999. PMID: 10574713
-
Lamin A/C speckles mediate spatial organization of splicing factor compartments and RNA polymerase II transcription.J Cell Biol. 2002 Dec 9;159(5):783-93. doi: 10.1083/jcb.200204149. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473687 Free PMC article.
-
Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies.Hum Mol Genet. 2004 Nov 1;13(21):2567-80. doi: 10.1093/hmg/ddh295. Epub 2004 Sep 14. Hum Mol Genet. 2004. PMID: 15367494
-
Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation.Annu Rev Biochem. 2015;84:131-64. doi: 10.1146/annurev-biochem-060614-034115. Epub 2015 Feb 26. Annu Rev Biochem. 2015. PMID: 25747401 Review.
-
Dysfunction of lamin A triggers a DNA damage response and cellular senescence.DNA Repair (Amst). 2006 Feb 3;5(2):286-9. doi: 10.1016/j.dnarep.2005.10.007. Epub 2005 Dec 15. DNA Repair (Amst). 2006. PMID: 16344005 Review.
Cited by
-
FUS interacts with nuclear matrix-associated protein SAFB1 as well as Matrin3 to regulate splicing and ligand-mediated transcription.Sci Rep. 2016 Oct 12;6:35195. doi: 10.1038/srep35195. Sci Rep. 2016. PMID: 27731383 Free PMC article.
-
Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice.Proc Natl Acad Sci U S A. 2011 Jul 26;108(30):12325-30. doi: 10.1073/pnas.1102789108. Epub 2011 Jul 11. Proc Natl Acad Sci U S A. 2011. PMID: 21746928 Free PMC article.
-
Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin.Genes Dev. 2008 Apr 1;22(7):832-53. doi: 10.1101/gad.1652708. Genes Dev. 2008. PMID: 18381888 Free PMC article. Review.
-
The metastasis efficiency modifier ribosomal RNA processing 1 homolog B (RRP1B) is a chromatin-associated factor.J Biol Chem. 2009 Oct 16;284(42):28660-73. doi: 10.1074/jbc.M109.023457. Epub 2009 Aug 26. J Biol Chem. 2009. PMID: 19710015 Free PMC article.
-
Exploring the Crosstalk Between LMNA and Splicing Machinery Gene Mutations in Dilated Cardiomyopathy.Front Genet. 2018 Jul 9;9:231. doi: 10.3389/fgene.2018.00231. eCollection 2018. Front Genet. 2018. PMID: 30050558 Free PMC article. Review.
References
-
- Bernhard, W. (1969). A new staining procedure for electron microscopical cytology. J. Ultrastruct. Res. 27, 250-265. - PubMed
-
- Bridger J.M., I.R. Kill, M. O.`Farrel, and C.J. Hutchinson. (1993). Internal lamin structures within G1 nuclei of dermal fibroblasts. J. Cell Sci. 104, 297-306. - PubMed
-
- Broers, J.L., Machiels, B.M., van Eys, G.J., Kuijpers, H.J., Manders, E.M., van Driel, R., and Ramaekers, F.C. (1999). Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J. Cell Sci. 112, 3463-3475. - PubMed
-
- Cohen, M., Lee, K.K., Wilson, K.L., and Gruenbaum, Y. (2001). Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem. Sci. 26, 41-47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

