Modulation of the biological activity of a tobacco LTP1 by lipid complexation
- PMID: 15356262
- PMCID: PMC524770
- DOI: 10.1091/mbc.e04-07-0575
Modulation of the biological activity of a tobacco LTP1 by lipid complexation
Abstract
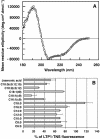
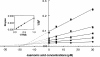
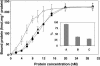
Plant lipid transfer proteins (LTPs) are small, cysteine-rich proteins secreted into the extracellular space. They belong to the pathogenesis-related proteins (PR-14) family and are believed to be involved in several physiological processes including plant disease resistance, although their precise biological function is still unknown. Here, we show that a recombinant tobacco LTP1 is able to load fatty acids and jasmonic acid. This LTP1 binds to specific plasma membrane sites, previously characterized as elicitin receptors, and is shown to be involved in the activation of plant defense. The biological properties of this LTP1 were compared with those of LTP1-linolenic and LTP1-jasmonic acid complexes. The binding curve of the LTP1-linolenic acid complex to purified tobacco plasma membranes is comparable to the curve obtained with LTP1. In contrast, the LTP1-jasmonic acid complex shows a strongly increased interaction with the plasma membrane receptors. Treatment of tobacco plants with LTP1-jasmonic acid resulted in an enhancement of resistance toward Phytophthora parasitica. These effects were absent upon treatment with LTP1 or jasmonic acid alone. This work presents the first evidence for a biological activity of a LTP1 and points out the crucial role of protein-specific lipophilic ligand interaction in the modulation of the protein activity.
Figures
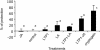
References
-
- Arondel, V., Vergnolle, C., Cantrel, C., and Kader, J.-C. (2000). Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Sci. 157, 1-12. - PubMed
-
- Balendiran, G.K., Schnütgen, F., Scapin, G., Börchers, T., Xhong, N., Lim, K., Godbout, R., Spener, F., and Sacchettini, J.C. (2000). Crystal structure and thermodynamic analysis of human brain fatty acid-binding protein. J. Biol. Chem. 275, 27045-27054. - PubMed
-
- Blée, E. (2002). Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 7, 315-322. - PubMed
-
- Blilou, I., Ocampo, J.A., and Garcia-Garrido, J.M. (2000). Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J. Exp. Bot. 51, 1969-1977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

