Marek's disease is a natural model for lymphomas overexpressing Hodgkin's disease antigen (CD30)
- PMID: 15356338
- PMCID: PMC518847
- DOI: 10.1073/pnas.0305789101
Marek's disease is a natural model for lymphomas overexpressing Hodgkin's disease antigen (CD30)
Abstract
Animal models are essential for elucidating the molecular mechanisms of carcinogenesis. Hodgkin's and many diverse non-Hodgkin's lymphomas overexpress the Hodgkin's disease antigen CD30 (CD30(hi)), a tumor necrosis factor receptor II family member. Here we show that chicken Marek's disease (MD) lymphoma cells are also CD30(hi) and are a unique natural model for CD30(hi) lymphoma. Chicken CD30 resembles an ancestral form, and we identify a previously undescribed potential cytoplasmic signaling domain conserved in chicken, human, and mouse CD30. Our phylogeneic analysis defines a relationship between the structures of human and mouse CD30 and confirms that mouse CD30 represents the ancestral mammalian gene structure. CD30 expression by MD virus (MDV)-transformed lymphocytes correlates with expression of the MDV Meq putative oncogene (a c-Jun homologue) in vivo. The chicken CD30 promoter has 15 predicted high-stringency Meq-binding transcription factor recognition motifs, and Meq enhances transcription from the CD30 promoter in vitro. Plasma proteomics identified a soluble form of CD30. CD30 overexpression is evolutionarily conserved and defines one class of neoplastic transformation events, regardless of etiology. We propose that CD30 is a component of a critical intracellular signaling pathway perturbed in neoplastic transformation. Specific anti-CD30 Igs occurred after infection of genetically MD-resistant chickens with oncogenic MDV, suggesting immunity to CD30 could play a role in MD lymphoma regression.
Figures


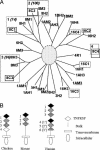

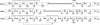
References
-
- Titcomb, C. P. J. (2001) J. Insur. Med. 33, 329–338. - PubMed
-
- National Cancer Institute Progress Review Group (2002) Strategic Plan for Addressing the Recommendations of the Leukemia, Lymphoma, and Myeloma Progress Review Group (National Institutes of Health, Bethesda).
-
- Chiarle, R., Podda, A., Prolla, G., Gong, J., Thorbecke, G. J. & Inghirami, G. (1999) Clin. Immunol. 90, 157–164. - PubMed
-
- Tarkowski, M. (1999) Arch. Immunol. Ther. Exp. (Warsz) 47, 217–221. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

