Efferent actions in the chinchilla vestibular labyrinth
- PMID: 15357416
- PMCID: PMC2538405
- DOI: 10.1007/s10162-003-4029-7
Efferent actions in the chinchilla vestibular labyrinth
Abstract
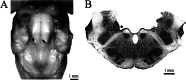
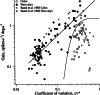
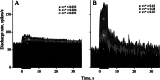
Efferent fibers were electrically stimulated in the brain stem, while afferent activity was recorded from the superior vestibular nerve in barbiturate-anesthetized chinchillas. We concentrated on canal afferents, but otolith afferents were also studied. Among canal fibers, calyx afferents were recognized by their irregular discharge and low rotational gains. In separate experiments, stimulating electrodes were placed in the efferent cell groups ipsilateral or contralateral to the recording electrode or in the midline. While single shocks were ineffective, repetitive shock trains invariably led to increases in afferent discharge rate. Such excitatory responses consisted of fast and slow components. Fast components were large only at high shock frequencies (200-333/s), built up with exponential time constants <0.1 s, and showed response declines or adaptation during shock trains >1 s in duration. Slow responses were obtained even at shock rates of 50/s, built up and decayed with time constants of 15-30 s, and could show little adaptation. The more regular the discharge, the larger was the efferent response of an afferent fiber. Response magnitude was proportional to cv*b, a normalized coefficient of interspike-interval variation (cv*) raised to the power b = 0.7. The value of the exponent b did not depend on unit type (calyx vs. bouton plus dimorphic, canal vs. otolith) or on stimulation site (ipsilateral, contralateral, or midline). Responses were slightly smaller with contralateral or midline stimulation than with ipsilateral stimulation, and they were smaller for otolith, as compared to canal, fibers. An anatomical study had suggested that responses to contralateral afferent stimulation should be small or nonexistent in irregular canal fibers. The suggestion was not confirmed in this study. Contralateral responses, including the large responses typically seen in irregular fibers, were abolished by shallow midline incisions that should have severed crossing efferent axons.
Figures


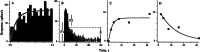




References
-
- Ashmore JF, Russell IJ. Effects of efferent nerve stimulation on hair cells of the frog sacculus. J. Physiol. 1982;329:25–26.
-
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J. Neurophysiol. 1988;60:182–203. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

