Contribution of Kir4.1 to the mouse electroretinogram
- PMID: 15359216
- PMCID: PMC2883771
Contribution of Kir4.1 to the mouse electroretinogram
Abstract
Purpose: The electroretinogram (ERG) represents the combination of several distinct cellular processes and conductances. Here, we define the contribution that K+ conductance through Kir4.1 channels makes to the mouse ERG.
Methods: To obtain mice expressing different levels of Kir4.1, we mated Kir4.1+/- mice and used PCR to identify Kir4.1+/- and Kir4.1+/+ littermates. In addition, we mated Kir4.1+/- males with females homozygous for the nob (no b-wave) defect, which eliminates post-receptoral contributions to the ERG. After overnight dark adaptation, mice were anesthetized and ERGs were recorded to 7 min stimuli, to focus on slow ERG components, or to strobe flash stimuli, to examine earlier ERG components.
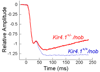
Results: The amplitudes of the ERG c-wave and the fast oscillation, measured from the c-wave peak, were significantly larger in Kir4.1+/- mice than in Kir4.1+/+ littermates. In comparison, the amplitude of the light peak, the other main component generated by the retinal pigment epithelium in response to light, did not differ between Kir4.1+/- and Kir4.1+/+ mice. The amplitude of slow PIII, revealed by the nob genetic background, was reduced in Kir4.1+/- mice.
Conclusions: These results indicate that a cornea-negative potential, generated by Kir4.1, normally opposes a positive polarity conductance that is generated by the apical membrane of the retinal pigment epithelium to form the c-wave measured at the corneal surface.
Figures



Similar articles
-
Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina.J Neurosci. 2000 Aug 1;20(15):5733-40. doi: 10.1523/JNEUROSCI.20-15-05733.2000. J Neurosci. 2000. PMID: 10908613 Free PMC article.
-
Evaluation of different recording parameters to establish a standard for flash electroretinography in rodents.Vision Res. 2001 Aug;41(17):2173-85. doi: 10.1016/s0042-6989(01)00103-1. Vision Res. 2001. PMID: 11448710
-
Early Functional and Morphologic Abnormalities in the Diabetic Nyxnob Mouse Retina.Invest Ophthalmol Vis Sci. 2016 Jun 1;57(7):3496-508. doi: 10.1167/iovs.15-18775. Invest Ophthalmol Vis Sci. 2016. PMID: 27367517 Free PMC article.
-
Human retinal dark adaptation tracked in vivo with the electroretinogram: insights into processes underlying recovery of cone- and rod-mediated vision.J Physiol. 2022 Nov;600(21):4603-4621. doi: 10.1113/JP283105. Epub 2022 Jun 7. J Physiol. 2022. PMID: 35612091 Free PMC article. Review.
-
Electroretinograms.Handb Clin Neurol. 2019;160:481-493. doi: 10.1016/B978-0-444-64032-1.00032-1. Handb Clin Neurol. 2019. PMID: 31277870 Review.
Cited by
-
Recommendations for a toxicological screening ERG procedure in laboratory animals.Doc Ophthalmol. 2005 Jan;110(1):57-66. doi: 10.1007/s10633-005-7344-y. Doc Ophthalmol. 2005. PMID: 16249957 Review.
-
Aquaporins and CFTR in ocular epithelial fluid transport.J Membr Biol. 2006 Mar;210(2):105-15. doi: 10.1007/s00232-005-0849-1. Epub 2006 Jul 25. J Membr Biol. 2006. PMID: 16868675 Review.
-
Progressive Cone-Rod Dystrophy and RPE Dysfunction in Mitfmi/+ Mice.Genes (Basel). 2023 Jul 17;14(7):1458. doi: 10.3390/genes14071458. Genes (Basel). 2023. PMID: 37510362 Free PMC article.
-
An unusual inherited electroretinogram feature with an exaggerated negative component in dogs.Vet Ophthalmol. 2022 Sep;25(5):385-397. doi: 10.1111/vop.12998. Epub 2022 Jun 17. Vet Ophthalmol. 2022. PMID: 35713167 Free PMC article.
-
Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome.J Physiol. 2011 Apr 1;589(Pt 7):1681-9. doi: 10.1113/jphysiol.2010.198531. Epub 2011 Feb 7. J Physiol. 2011. PMID: 21300747 Free PMC article.
References
-
- Armington JC. The electroretinogram. New York: Academic Press; 1974.
-
- Fishman GA, Birch DG, Holder GE, Brigell MG, editors. Electrophysiologic testing in disorders of the retina, optic nerve, and visual pathway. 2nd ed. San Francisco: American Academy of Ophthalmology; 2001.
-
- Peachey NS, Ball SL. Electrophysiological analysis of visual function in mutant mice. Doc Ophthalmol. 2003;107:13–36. - PubMed
-
- Granit R. Sensory mechanisms of the retina, with an appendix on electroretinography. London: Oxford University Press; 1947.
-
- Steinberg RH, Linsenmeier RA, Griff ER. Retinal pigment epithelial cell contributions to the electroretinogram and electrooculogram. In: Osborne NN, Chader GJ, editors. Progress in retinal research. New York: Pergamon Press; 1985. pp. 33–66.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

