Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM
- PMID: 15359281
- PMCID: PMC522792
- DOI: 10.1038/sj.emboj.7600393
Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM
Abstract
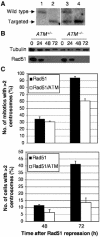
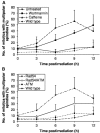
Centrosomes are the principal microtubule organising centres in somatic cells. Abnormal centrosome number is common in tumours and occurs after gamma-irradiation and in cells with mutations in DNA repair genes. To investigate how DNA damage causes centrosome amplification, we examined cells that conditionally lack the Rad51 recombinase and thereby incur high levels of spontaneous DNA damage. Rad51-deficient cells arrested in G2 phase and formed supernumerary functional centrosomes, as assessed by light and serial section electron microscopy. This centrosome amplification occurred without an additional DNA replication round and was not the result of cytokinesis failure. G2-to-M checkpoint over-ride by caffeine or wortmannin treatment strongly reduced DNA damage-induced centrosome amplification. Radiation-induced centrosome amplification was potentiated by Rad54 disruption. Gene targeting of ATM reduced, but did not abrogate, centrosome amplification induced by DNA damage in both the Rad51 and Rad54 knockout models, demonstrating ATM-dependent and -independent components of DNA damage-inducible G2-phase centrosome amplification. Our data suggest DNA damage-induced centrosome amplification as a mechanism for ensuring death of cells that evade the DNA damage or spindle assembly checkpoints.
Figures
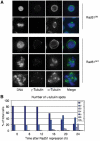


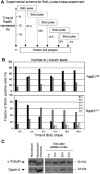
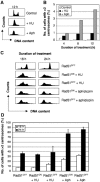
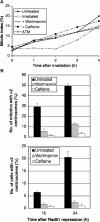
Similar articles
-
Roles of nibrin and AtM/ATR kinases on the G2 checkpoint under endogenous or radio-induced DNA damage.Biol Res. 2005;38(2-3):179-85. doi: 10.4067/s0716-97602005000200007. Biol Res. 2005. PMID: 16238096
-
The controlling role of ATM in homologous recombinational repair of DNA damage.EMBO J. 2000 Feb 1;19(3):463-71. doi: 10.1093/emboj/19.3.463. EMBO J. 2000. PMID: 10654944 Free PMC article.
-
Activation of the S phase DNA damage checkpoint by mitomycin C.J Cell Physiol. 2007 May;211(2):468-76. doi: 10.1002/jcp.20957. J Cell Physiol. 2007. PMID: 17167777
-
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review.
-
The impact of a negligent G2/M checkpoint on genomic instability and cancer induction.Nat Rev Cancer. 2007 Nov;7(11):861-9. doi: 10.1038/nrc2248. Nat Rev Cancer. 2007. PMID: 17943134 Review.
Cited by
-
Binding between ROCK1 and DCTN2 triggers diabetes‑associated centrosome amplification in colon cancer cells.Oncol Rep. 2021 Jul;46(1):151. doi: 10.3892/or.2021.8102. Epub 2021 Jun 3. Oncol Rep. 2021. PMID: 34080666 Free PMC article.
-
A novel role of human holliday junction resolvase GEN1 in the maintenance of centrosome integrity.PLoS One. 2012;7(11):e49687. doi: 10.1371/journal.pone.0049687. Epub 2012 Nov 16. PLoS One. 2012. PMID: 23166748 Free PMC article.
-
Moonlighting at the Poles: Non-Canonical Functions of Centrosomes.Front Cell Dev Biol. 2022 Jul 14;10:930355. doi: 10.3389/fcell.2022.930355. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912107 Free PMC article. Review.
-
p53 controls genomic stability and temporal differentiation of human neural stem cells and affects neural organization in human brain organoids.Cell Death Dis. 2020 Jan 23;11(1):52. doi: 10.1038/s41419-019-2208-7. Cell Death Dis. 2020. PMID: 31974372 Free PMC article.
-
Centrosomes and associated proteins in pathogenesis and treatment of breast cancer.Front Oncol. 2024 Mar 28;14:1370565. doi: 10.3389/fonc.2024.1370565. eCollection 2024. Front Oncol. 2024. PMID: 38606093 Free PMC article. Review.
References
-
- Abraham RT (2001) Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 - PubMed
-
- Bertrand P, Lambert S, Joubert C, Lopez BS (2003) Overexpression of mammalian Rad51 does not stimulate tumorigenesis while a dominant-negative Rad51 affects centrosome fragmentation, ploidy and stimulates tumorigenesis, in p53-defective CHO cells. Oncogene 22: 7587–7592 - PubMed
-
- Bezzubova O, Silbergleit A, Yamaguchi-Iwai Y, Takeda S, Buerstedde JM (1997) Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell 89: 185–193 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

