Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract
- PMID: 15361072
- PMCID: PMC1134114
- DOI: 10.1042/BJ20040605
Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract
Abstract
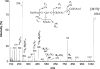
Purified human mucins from different parts of the intestinal tract (ileum, cecum, transverse and sigmoid colon and rectum) were isolated from two individuals with blood group ALe(b) (A-Lewis(b)). After alkaline borohydride treatment the released oligosaccharides were structurally characterized by nano-ESI Q-TOF MS/MS (electrospray ionization quadrupole time-of-flight tandem MS) without prior fractionation or derivatization. More than 100 different oligosaccharides, with up to ten monosaccharide residues, were identified using this technique. Oligosaccharides based on core 3 structures, GlcNAc(beta1-3)GalNAc (where GlcNAc is N-acetyl-D-glucosamine and GalNAc is N-acetylgalactosamine), were widely distributed in human intestinal mucins. Core 5 structures, GalNAc(alpha1-3)GalNAc, were also recovered in all fractions. Moreover, a comparison of the oligosaccharide repertoire, with respect to size, diversity and expression of glycans and terminal epitopes, showed a high level of mucin-specific glycosylation: highly fucosylated glycans, found specifically in the small intestine, were mainly based on core 4 structures, GlcNAc-(beta1-3)[GlcNAc(beta1-6)]GalNAc, whereas the sulpho-Le(X) determinant carrying core 2 glycans, Gal(beta1-3)[GlcNAc(beta1-6)]-GalNAc (where Gal is galactose), was recovered mainly in the distal colon. Blood group H and A antigenic determinants were present exclusively in the ileum and cecum, whereas blood group Sd(a)/Cad related epitopes, GalNAc(beta1-4)[NeuAc(alpha2-3)]Gal (where NeuAc is N-acetylneuraminate), were found to increase along the length of the colon. Our findings suggest that mucins create an enormous repertoire of potential binding sites for micro-organisms that could explain the regio-specific colonization of bacteria in the human intestinal tract.
Figures





Comment in
-
Intestinal candyfloss.Biochem J. 2004 Dec 1;384(Pt 2):e3-5. doi: 10.1042/BJ20041655. Biochem J. 2004. PMID: 15549969 Free PMC article. Review.
References
-
- Allen A. Mucus – a protective secretion of complexity. Trends Biochem. Sci. 1983;8:169–173.
-
- Neutra M. R., Forstner J. F. Gastrointestinal mucus: synthesis, secretion and function. In: Johnson L. R., editor. Physiology of the Gastrointestinal Tract. 2nd edn. New York: Raven Press; 1987. pp. 975–1009.
-
- Strous G. J., Dekker J. Mucin-type glycoprotein. J. Crit. Rev. Biochem. Mol. Biol. 1992;27:57–92. - PubMed
-
- Forstner J. F., Forstner G. G. Gastrointestinal mucus. In: Johnson L. R., editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1245–1283.
-
- Gum J. R. Human mucin glycoproteins: varied structures predict diverse properties and specific functions. Biochem. Soc. Trans. 1995;23:795–799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

