The high concentration of Arg213-->Gly extracellular superoxide dismutase (EC-SOD) in plasma is caused by a reduction of both heparin and collagen affinities
- PMID: 15362977
- PMCID: PMC1134713
- DOI: 10.1042/BJ20041218
The high concentration of Arg213-->Gly extracellular superoxide dismutase (EC-SOD) in plasma is caused by a reduction of both heparin and collagen affinities
Abstract
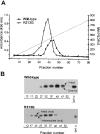
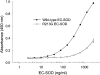
The C-terminal region of EC-SOD (extracellular superoxide dismutase) mediates the binding to both heparin/heparan sulphate and type I collagen. A mutation (Arg213-->Gly; R213G) within this extracellular matrix-binding region has recently been implicated in the development of heart disease. This relatively common mutation affects the heparin affinity, and the concentration of EC-SOD in the plasma of R213G homozygous individuals is increased 10- to 30-fold. In the present study we confirm, using R213G EC-SOD purified from a homozygous individual, that the heparin affinity is reduced. Significantly, the collagen affinity of the R213G EC-SOD variant was similarly affected and both the heparin and collagen affinities were reduced by 12-fold. Structural analysis of synthetic extracellular matrix-binding regions suggests that the mutation alters the secondary structure. We conclude that the increased concentration of EC-SOD in the plasma of R213G carriers is caused by a reduction in both heparin and collagen affinities.
Figures


References
-
- Fattman C. L., Schaefer L. M., Oury T. D. Extracellular superoxide dismutase in biology and medicine. Free Radical Biol. Med. 2003;35:236–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

