Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest
- PMID: 15364958
- PMCID: PMC2172115
- DOI: 10.1083/jcb.200405128
Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest
Abstract
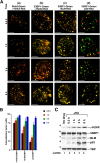
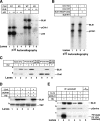
Bloom's syndrome is a rare autosomal recessive genetic disorder characterized by chromosomal aberrations, genetic instability, and cancer predisposition, all of which may be the result of abnormal signal transduction during DNA damage recognition. Here, we show that BLM is an intermediate responder to stalled DNA replication forks. BLM colocalized and physically interacted with the DNA damage response proteins 53BP1 and H2AX. Although BLM facilitated physical interaction between p53 and 53BP1, 53BP1 was required for efficient accumulation of both BLM and p53 at the sites of stalled replication. The accumulation of BLM/53BP1 foci and the physical interaction between them was independent of gamma-H2AX. The active Chk1 kinase was essential for both the accurate focal colocalization of 53BP1 with BLM and the consequent stabilization of BLM. Once the ATR/Chk1- and 53BP1-mediated signal from replicational stress is received, BLM functions in multiple downstream repair processes, thereby fulfilling its role as a caretaker tumor suppressor.
Figures




References
-
- Abraham, R.T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177–2196. - PubMed
-
- Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 3:421–429. - PubMed
-
- Beamish, H., P. Kedar, H. Kaneko, P. Chen, T. Fukao, C. Peng, S. Beresten, N. Gueven, D. Purdie, S. Lees-Miller, et al. 2002. Functional link between BLM defective in Bloom's syndrome and the ataxia-telangiectasia-mutated protein, ATM. J. Biol. Chem. 277:30515–30523. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

