Catch bonds govern adhesion through L-selectin at threshold shear
- PMID: 15364963
- PMCID: PMC2172126
- DOI: 10.1083/jcb.200403144
Catch bonds govern adhesion through L-selectin at threshold shear
Erratum in
- J Cell Biol. 2005 Mar 14;168(6):975
Abstract
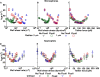
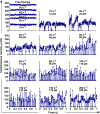
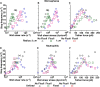
Flow-enhanced cell adhesion is an unexplained phenomenon that might result from a transport-dependent increase in on-rates or a force-dependent decrease in off-rates of adhesive bonds. L-selectin requires a threshold shear to support leukocyte rolling on P-selectin glycoprotein ligand-1 (PSGL-1) and other vascular ligands. Low forces decrease L-selectin-PSGL-1 off-rates (catch bonds), whereas higher forces increase off-rates (slip bonds). We determined that a force-dependent decrease in off-rates dictated flow-enhanced rolling of L-selectin-bearing microspheres or neutrophils on PSGL-1. Catch bonds enabled increasing force to convert short-lived tethers into longer-lived tethers, which decreased rolling velocities and increased the regularity of rolling steps as shear rose from the threshold to an optimal value. As shear increased above the optimum, transitions to slip bonds shortened tether lifetimes, which increased rolling velocities and decreased rolling regularity. Thus, force-dependent alterations of bond lifetimes govern L-selectin-dependent cell adhesion below and above the shear optimum. These findings establish the first biological function for catch bonds as a mechanism for flow-enhanced cell adhesion.
Figures




Similar articles
-
Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan.J Biol Chem. 2004 Jan 16;279(3):2291-8. doi: 10.1074/jbc.M310396200. Epub 2003 Oct 22. J Biol Chem. 2004. PMID: 14573602
-
Enhancement of L-selectin, but not P-selectin, bond formation frequency by convective flow.Biophys J. 2008 Feb 1;94(3):1034-45. doi: 10.1529/biophysj.106.098707. Epub 2007 Sep 21. Biophys J. 2008. PMID: 17890384 Free PMC article.
-
Flow-enhanced adhesion regulated by a selectin interdomain hinge.J Cell Biol. 2006 Sep 25;174(7):1107-17. doi: 10.1083/jcb.200606056. J Cell Biol. 2006. PMID: 17000883 Free PMC article.
-
Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings.Mol Immunol. 2013 Aug;55(1):59-69. doi: 10.1016/j.molimm.2012.10.025. Epub 2012 Nov 9. Mol Immunol. 2013. PMID: 23141302 Free PMC article. Review.
-
Biomechanics of leukocyte rolling.Biorheology. 2011;48(1):1-35. doi: 10.3233/BIR-2011-0579. Biorheology. 2011. PMID: 21515934 Free PMC article. Review.
Cited by
-
Quantitative interpretation of cell rolling velocity distribution.Biophys J. 2021 Jun 15;120(12):2511-2520. doi: 10.1016/j.bpj.2021.04.021. Epub 2021 Apr 29. Biophys J. 2021. PMID: 33932434 Free PMC article.
-
Distinct kinetic and molecular requirements govern CD44 binding to hyaluronan versus fibrin(ogen).Biophys J. 2012 Aug 8;103(3):415-423. doi: 10.1016/j.bpj.2012.06.039. Biophys J. 2012. PMID: 22947857 Free PMC article.
-
Catch-bond model derived from allostery explains force-activated bacterial adhesion.Biophys J. 2006 Feb 1;90(3):753-64. doi: 10.1529/biophysj.105.066548. Epub 2005 Nov 4. Biophys J. 2006. PMID: 16272438 Free PMC article.
-
Neutrophils-From Bone Marrow to First-Line Defense of the Innate Immune System.Front Immunol. 2021 Dec 23;12:767175. doi: 10.3389/fimmu.2021.767175. eCollection 2021. Front Immunol. 2021. PMID: 35003081 Free PMC article. Review.
-
Regulation of catch bonds by rate of force application.J Biol Chem. 2011 Sep 16;286(37):32749-61. doi: 10.1074/jbc.M111.240044. Epub 2011 Jul 20. J Biol Chem. 2011. PMID: 21775439 Free PMC article.
References
-
- Alon, R., D.A. Hammer, and T.A. Springer. 1995. Lifetime of the P-selectin: carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. - PubMed
-
- Bell, G.I. 1978. Models for the specific adhesion of cells to cells. Science. 200:618–627. - PubMed
-
- Bruehl, R.E., T.A. Springer, and D.F. Bainton. 1996. Quantitation of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J. Histochem. Cytochem. 44:835–844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

