Identification to the species level and differentiation between strains of Aspergillus clinical isolates by automated repetitive-sequence-based PCR
- PMID: 15364984
- PMCID: PMC516350
- DOI: 10.1128/JCM.42.9.4016-4024.2004
Identification to the species level and differentiation between strains of Aspergillus clinical isolates by automated repetitive-sequence-based PCR
Abstract
A commercially available repetitive-sequence-based PCR (rep-PCR) DNA fingerprinting assay adapted to an automated format, the DiversiLab system, enables rapid microbial identification and strain typing. We explored the performance of the DiversiLab system as a molecular typing tool for 69 Aspergillus isolates (38 A. fumigatus, 15 A. flavus, and 16 A. terreus isolates) had been previously characterized by morphological analysis. Initially, 27 Aspergillus isolates (10 A. fumigatus, 9 A. flavus, and 8 A. terreus isolates) were used as controls to create a rep-PCR-based DNA fingerprint library with the DiversiLab software. Then, 42 blinded Aspergillus isolates were typed using the system. The rep-PCR-based profile revealed 98% concordance with morphology-based identification. rep-PCR-based DNA fingerprints were reproducible and were consistent for DNA from both hyphae and conidia. DiversiLab dendrogram reports correctly identified all A. fumigatus (n = 28), A. terreus (n = 8), and A. flavus (n = 6) isolates in the 42 blinded Aspergillus isolates. rep-PCR-based identification of all isolates was 100% in agreement with the contiguous internal transcribed spacer (ITS) region (ITS1-5.8S-ITS2) sequence-based identification of the respective isolates. Additionally, the DiversiLab system could demonstrate strain-level differentiation of A. flavus and A. terreus. Automated rep-PCR may be a time-efficient, effective, easy-to-use, novel genotyping tool for identifying and determining the strain relatedness of fungi. This system may be useful for epidemiological studies, molecular typing, and surveillance of Aspergillus species.
Figures
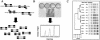


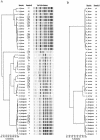

References
-
- Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. - PubMed
-
- Bart-Delabesse, E., C. Cordonnier, and S. Bretagne. 1999. Usefulness of genotyping with microsatellite markers to investigate hospital-acquired invasive aspergillosis. J. Hosp. Infect. 42:321-327. - PubMed
-
- Bart-Delabesse, E., J. Sarfati, J. P. Debeaupuis, W. van Leeuwen, A. van Belkum, S. Bretagne, and J. P. Latge. 2001. Comparison of restriction fragment length polymorphism, microsatellite length polymorphism, and random amplification of polymorphic DNA analyses for fingerprinting Aspergillus fumigatus isolates. J. Clin. Microbiol. 39:2683-2686. - PMC - PubMed
-
- Bertout, S., F. Renaud, T. De Meeus, M. A. Piens, B. Lebeau, M. A. Viviani, M. Mallie, J. M. Bastide, et al. 2000. Multilocus enzyme electrophoresis analysis of Aspergillus fumigatus strains isolated from the first clinical sample from patients with invasive aspergillosis. J. Med. Microbiol. 49:375-381. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

